Abstract
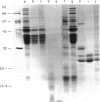
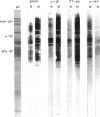
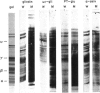
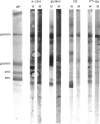
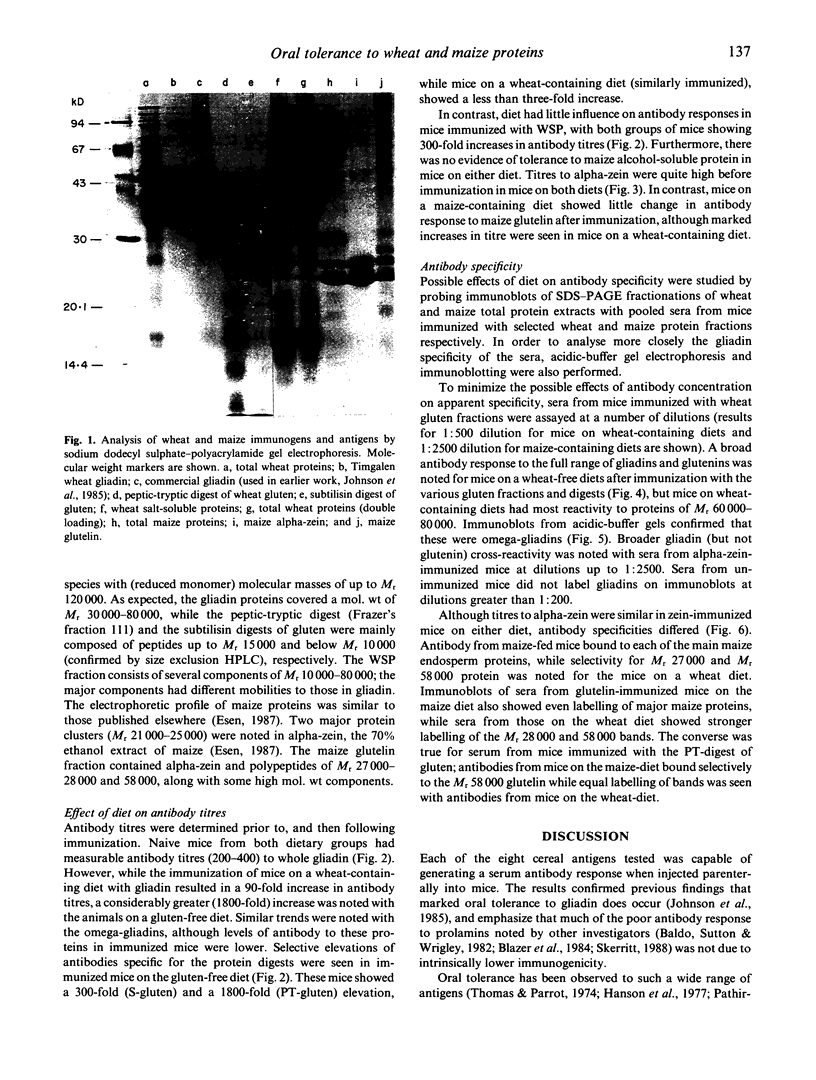
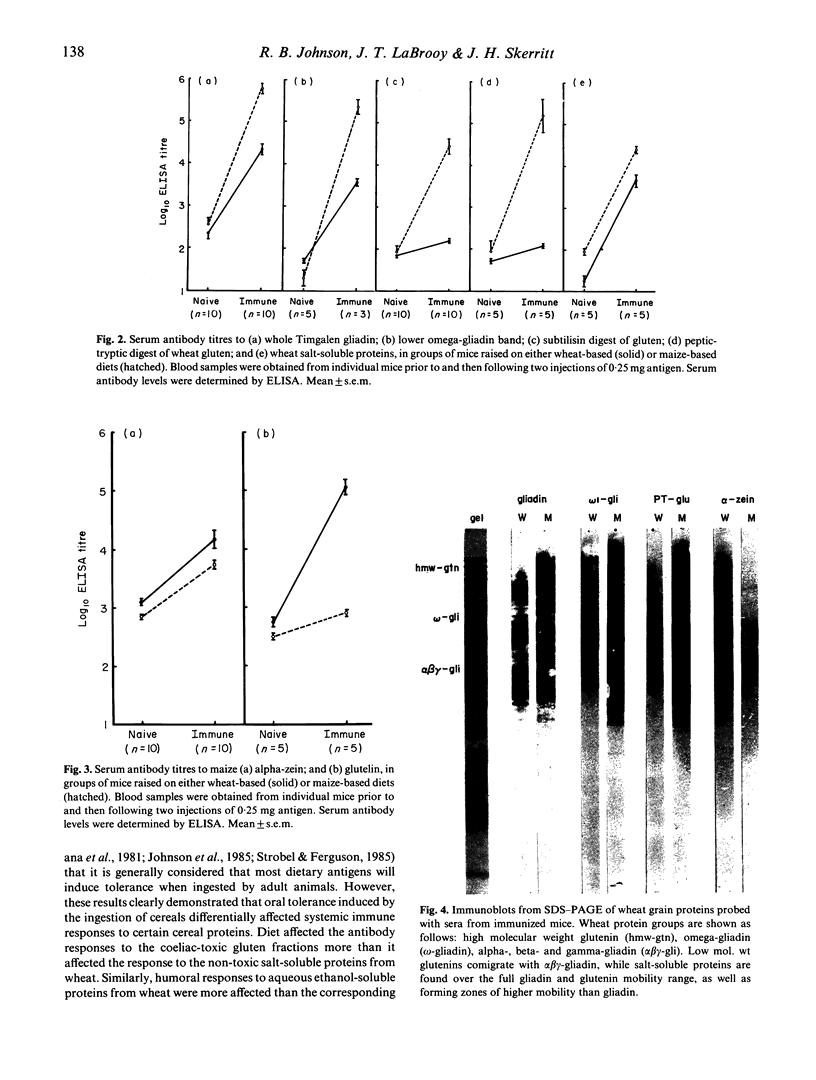
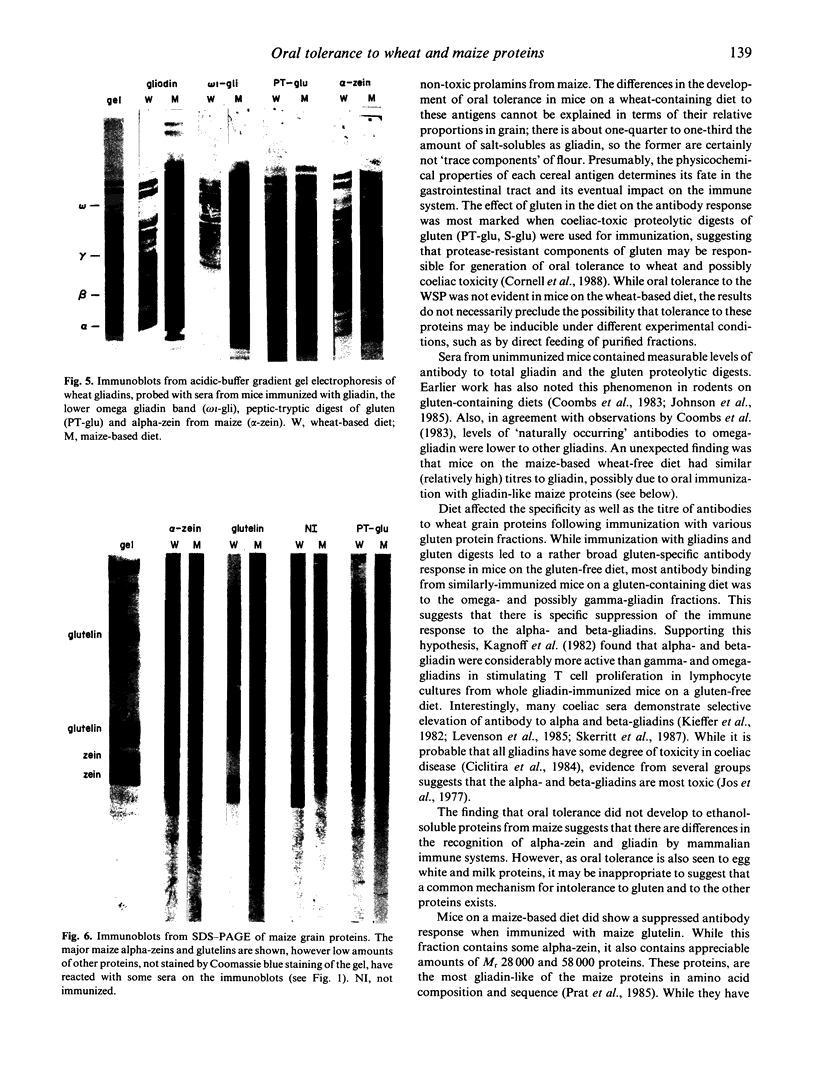
The influence of diet on humoral immune responses to gluten- and maize-derived proteins was examined using ELISA and protein blotting techniques. Mice raised on the maize-based (gluten-free) diet responded well to parenteral immunization with each of six gluten-derived protein preparations (whole gliadin, two omega-gliadin fractions, wheat salt-soluble proteins, a peptic-tryptic digest and a subtilisin digest of gluten), as serum antibody levels increased at least 300-fold in each case. In contrast, mice raised on the wheat-based diet responded poorly to immunization with either whole gliadin or omega-gliadin and were virtually non-responsive to enzymic digest of gluten. Diet had little effect on the magnitude of the antibody response to wheat salt-soluble proteins, with both groups showing a 300-fold increase in titre. Similarly, tolerance to alpha-zeins, the alcohol-soluble proteins of maize, did not occur on either diet. However, some oral tolerance was observed to maize glutelin. The specificity of the various antibody responses was then analysed by immunoblotting. Following immunization with gluten proteins or digests, antibodies from the maize-fed mice bound more or less equally to each of the main gliadin bands and to the glutenins while the mice on the wheat-based diet had antibody specific for omega-gliadin proteins. Serum antibodies from the maize-fed mice, immunized with either alpha-zein or maize glutelin, showed even labelling of the major maize endosperm proteins while antibodies from mice on the wheat diet showed strong labelling of the Mr 27,000 and 58,000 bands. These results show that diet influenced the specificity, as well as the magnitude of serum antibody responses to cereal proteins. In addition, oral tolerance appeared to affect the humoral response to some cereal proteins more than others. Both of these findings have important implications for our understanding of coeliac disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anand B. S., Piris J., Truelove S. C. The role of various cereals in coeliac disease. Q J Med. 1978 Jan;47(185):101–110. [PubMed] [Google Scholar]
- Baldo B. A., Sutton R., Wrigley C. W. Grass allergens, with particular reference to cereals. Prog Allergy. 1982;30:1–66. [PubMed] [Google Scholar]
- Blazer S., Naveh Y., Berant M., Merzbach D., Sperber S. Serum IgG antibodies to gliadin in children with celiac disease as measured by an immunofluorescence method. J Pediatr Gastroenterol Nutr. 1984 Mar;3(2):205–209. doi: 10.1097/00005176-198403000-00006. [DOI] [PubMed] [Google Scholar]
- Ciclitira P. J., Evans D. J., Fagg N. L., Lennox E. S., Dowling R. H. Clinical testing of gliadin fractions in coeliac patients. Clin Sci (Lond) 1984 Mar;66(3):357–364. doi: 10.1042/cs0660357. [DOI] [PubMed] [Google Scholar]
- Cole S. G., Kagnoff M. F. Celiac disease. Annu Rev Nutr. 1985;5:241–266. doi: 10.1146/annurev.nu.05.070185.001325. [DOI] [PubMed] [Google Scholar]
- Coombs R. R., Kieffer M., Fraser D. R., Frazier P. J. Naturally developing antibodies to wheat gliadin fractions and to other cereal antigens in rabbits, rats and guinea pigs on normal laboratory diets. Int Arch Allergy Appl Immunol. 1983 Mar;70(3):200–204. doi: 10.1159/000233323. [DOI] [PubMed] [Google Scholar]
- Cornell H. J., Auricchio R. S., De Ritis G., De Vincenzi M., Maiuri L., Raia V., Silano V. Intestinal mucosa of celiacs in remission is unable to abolish toxicity of gliadin peptides on in vitro developing fetal rat intestine and cultured atrophic celiac mucosa. Pediatr Res. 1988 Aug;24(2):233–237. doi: 10.1203/00006450-198808000-00019. [DOI] [PubMed] [Google Scholar]
- Davidson A. G., Bridges M. A. Coeliac disease: a critical review of aetiology and pathogenesis. Clin Chim Acta. 1987 Feb 27;163(1):1–40. doi: 10.1016/0009-8981(87)90031-3. [DOI] [PubMed] [Google Scholar]
- FRAZER A. C., FLETCHER R. F., ROSS C. A., SHAW B., SAMMONS H. G., SCHNEIDER R. Gluten-induced enteropathy: the effect of partially digested gluten. Lancet. 1959 Sep 5;2(7097):252–255. doi: 10.1016/s0140-6736(59)92051-3. [DOI] [PubMed] [Google Scholar]
- Hanson D. G., Vaz N. M., Maia L. C., Hornbrook M. M., Lynch J. M., Roy C. A. Inhibition of specific immune responses by feeding protein antigens. Int Arch Allergy Appl Immunol. 1977;55(1-6):526–532. doi: 10.1159/000231966. [DOI] [PubMed] [Google Scholar]
- Johnson R. B., LaBrooy J. T., Shearman D. J., Davidson G. P. The effect of diet on systemic immune responses to wheat gliadin. Aust J Exp Biol Med Sci. 1985 Jun;63(Pt 3):299–304. doi: 10.1038/icb.1985.33. [DOI] [PubMed] [Google Scholar]
- Kagnoff M. F., Austin R. K., Johnson H. C., Bernardin J. E., Dietler M. D., Kasarda D. D. Celiac sprue: correlation with murine T cell responses to wheat gliadin components. J Immunol. 1982 Dec;129(6):2693–2697. [PubMed] [Google Scholar]
- Korenblat P. E., Rothberg R. M., Minden P., Farr R. S. Immune responses of human adults after oral and parenteral exposure to bovine serum albumin. J Allergy. 1968 Apr;41(4):226–235. doi: 10.1016/0021-8707(68)90046-4. [DOI] [PubMed] [Google Scholar]
- Labrooy J. T., Hohmann A. W., Davidson G. P., Hetzel P. A., Johnson R. B., Shearman D. J. Intestinal and serum antibody in coeliac disease: a comparison using ELISA. Clin Exp Immunol. 1986 Dec;66(3):661–668. [PMC free article] [PubMed] [Google Scholar]
- Levenson S. D., Austin R. K., Dietler M. D., Kasarda D. D., Kagnoff M. F. Specificity of antigliadin antibody in celiac disease. Gastroenterology. 1985 Jul;89(1):1–5. doi: 10.1016/0016-5085(85)90737-1. [DOI] [PubMed] [Google Scholar]
- O'Farrelly C., Whelan C. A., Feighery C. F., Weir D. G. Suppressor-cell activity in coeliac disease induced by alpha-gliadin, a dietary antigen. Lancet. 1984 Dec 8;2(8415):1305–1307. doi: 10.1016/s0140-6736(84)90822-5. [DOI] [PubMed] [Google Scholar]
- Pathirana C., Goulding N. J., Gibney M. J., Pitts J. M., Gallagher P. J., Taylor T. G. Immune tolerance produced by pre- and postnatal exposure to dietary antigens. Int Arch Allergy Appl Immunol. 1981;66(1):114–118. doi: 10.1159/000232808. [DOI] [PubMed] [Google Scholar]
- Prat S., Cortadas J., Puigdomènech P., Palau J. Nucleic acid (cDNA) and amino acid sequences of the maize endosperm protein glutelin-2. Nucleic Acids Res. 1985 Mar 11;13(5):1493–1504. doi: 10.1093/nar/13.5.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerritt J. H., Johnson R. B., Hetzel P. A., La Brooy J. T., Shearman D. J., Davidson G. P. Variation of serum and intestinal gluten antibody specificities in coeliac disease. Clin Exp Immunol. 1987 Apr;68(1):189–199. [PMC free article] [PubMed] [Google Scholar]
- Strobel S., Ferguson A. Oral tolerance--induction and modulation. Klin Padiatr. 1985 Jul-Aug;197(4):297–301. doi: 10.1055/s-2008-1033987. [DOI] [PubMed] [Google Scholar]
- VAN DE KAMER J. H., WEIJERS H. A., DICKE W. K. Coeliac disease. IV. An investigation into the injurious constituents of wheat in connection with their action on patients with coeliac disease. Acta Paediatr. 1953 May;42(3):223–231. doi: 10.1111/j.1651-2227.1953.tb05586.x. [DOI] [PubMed] [Google Scholar]
- Warshaw A. L., Walker W. A., Cornell R., Isselbacher K. J. Small intestinal permeability to macromolecules. Transmission of horseradish peroxidase into mesenteric lymph and portal blood. Lab Invest. 1971 Dec;25(6):675–684. [PubMed] [Google Scholar]