Abstract
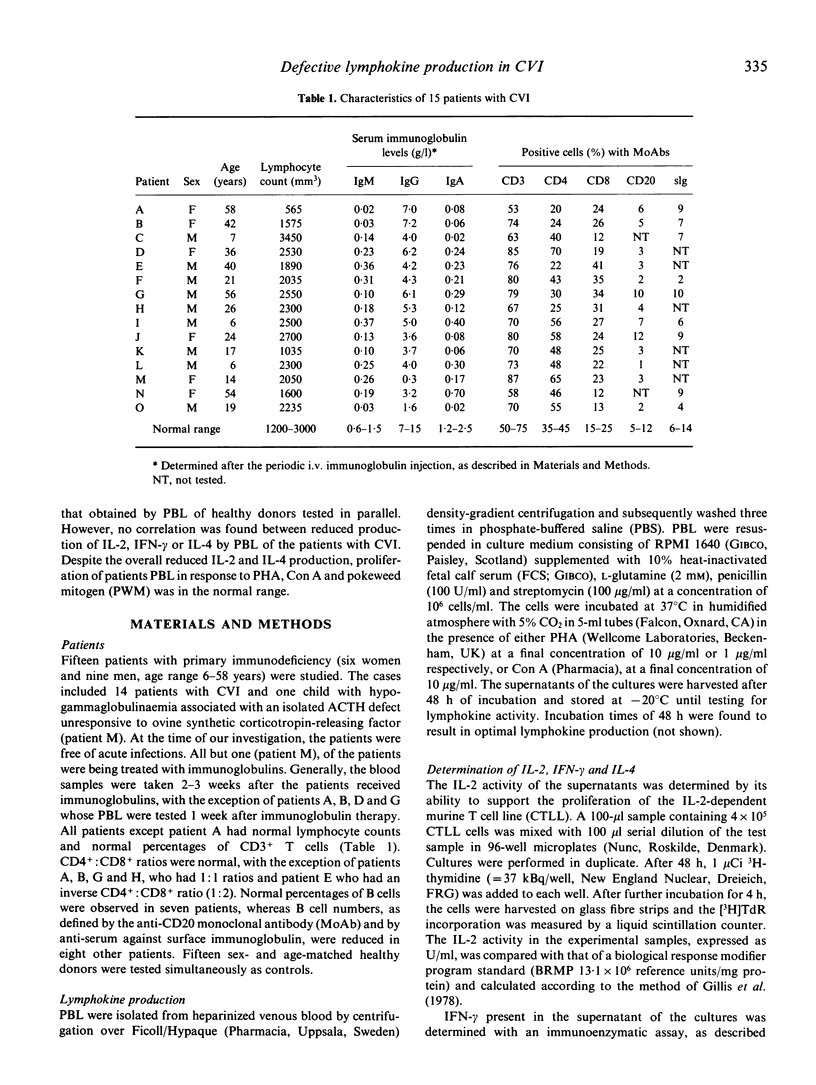
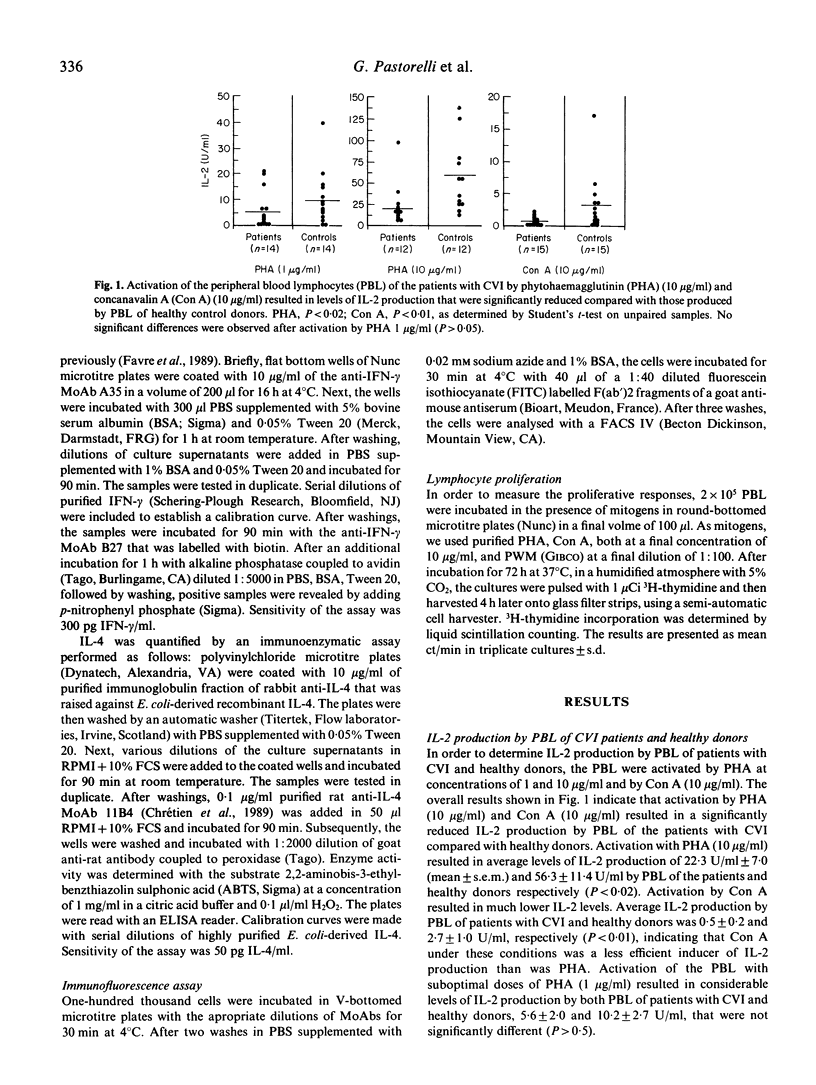
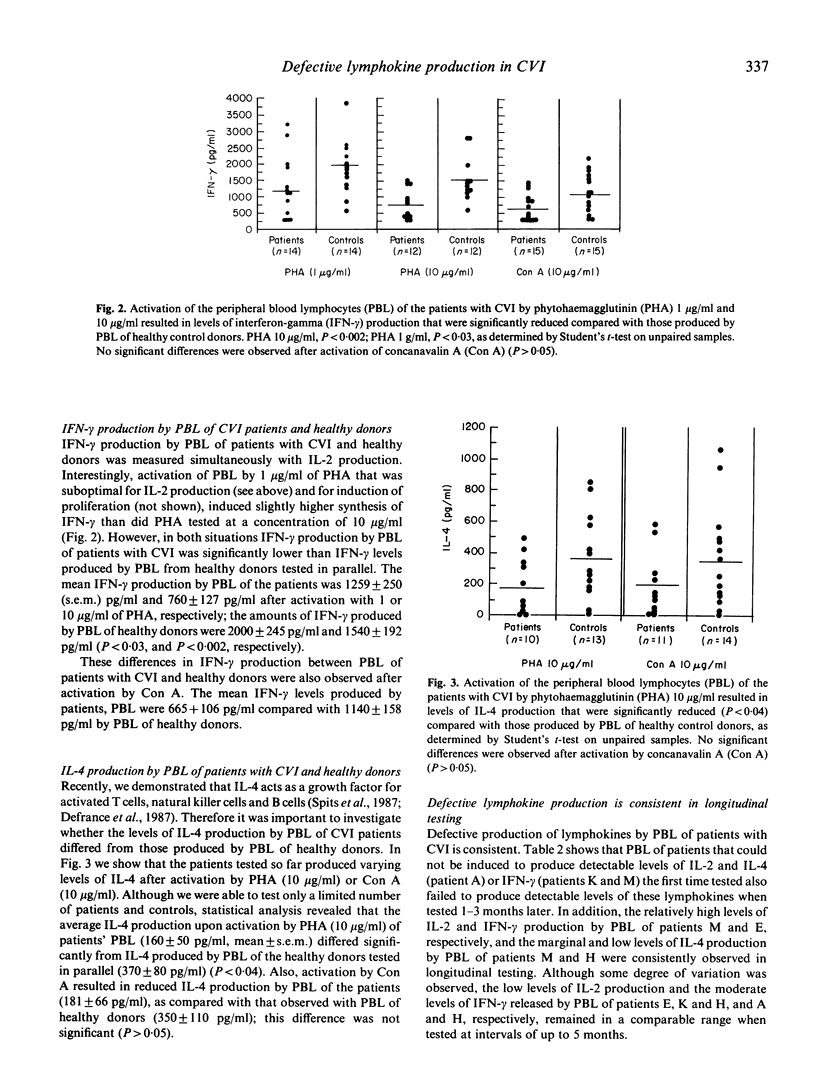
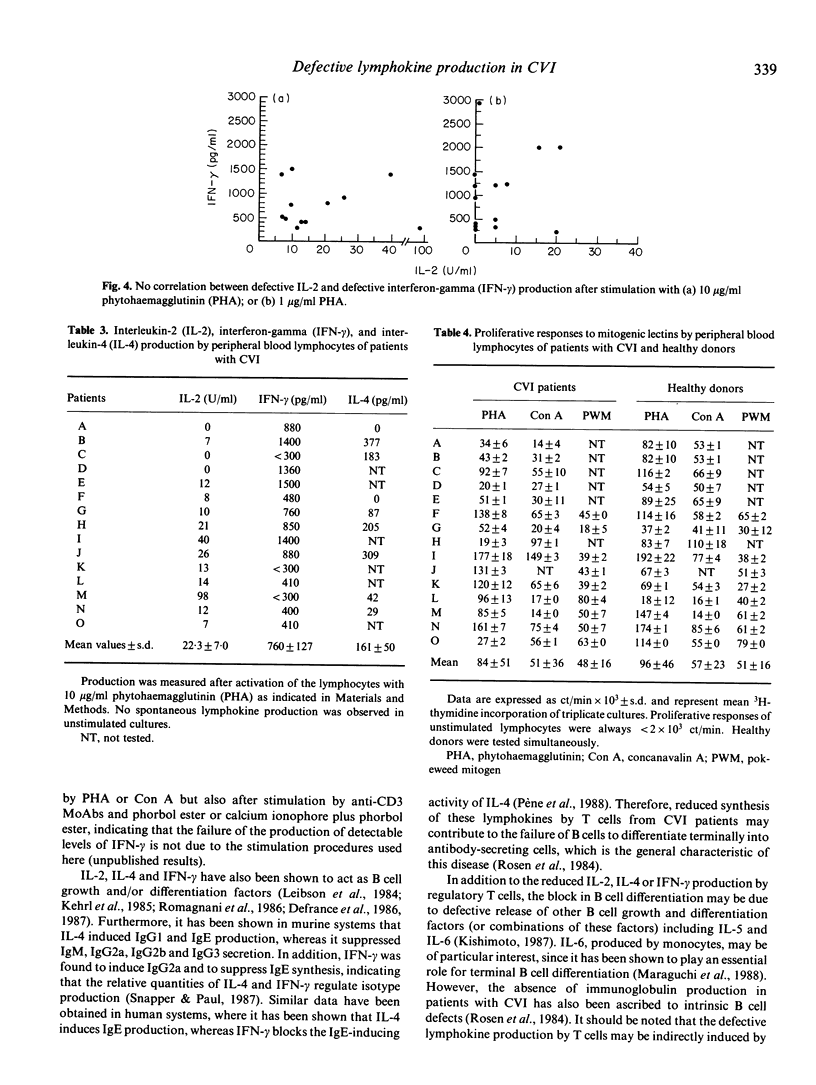
Peripheral blood lymphocytes (PBL) of 11 patients with CVI produced reduced levels of interleukin-4 (IL-4) upon activation by mitogens as compared with those secreted by PBL of healthy donors. The interleukin-2 (IL-2) and interferon-gamma (IFN-gamma) production by PBL of a series of 15 patients with CVI was also reduced. Decreased levels of IL-4 or IL-2 and IFN-gamma production were not only observed after activation by phytohaemagglutinin (PHA) at concentrations of 10 and 1 micrograms/ml, but also after activation by concanavalin A (Con A, 10 micrograms/ml). Longitudinal studies indicated that this defective lymphokine production was consistent upon testing periods up to 5 months. No correlation between reduced IL-4, IL-2 or IFN-gamma production was observed. PBL of patients that produced reduced levels of one lymphokine generally secreted normal levels of the other two lymphokines. Despite the reduced synthesis of the T cell growth factors IL-2 and IL-4, the proliferative responses of the PBL of the patients were in the normal range, which is compatible with the finding that IL-2 and IL-4 have synergistic effects on lymphocyte proliferation, particularly when one of these lymphokines is present at suboptimal concentrations. Since IL-2, IL-4 and IFN-gamma can act as B cell growth and differentiation factors, our data suggest that the reduced synthesis of these lymphokines may contribute to the deficient immunoglobulin production in patients with CVI.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Callard R. E., Shields J. G., Smith S. H., Lau Y. L., Levinsky R. J. Measurement of human B cell responses to growth and differentiation factors: relevance for immunodeficiency disease. Lymphokine Res. 1986;5 (Suppl 1):S151–S156. [PubMed] [Google Scholar]
- Chrétien I., Van Kimmenade A., Pearce M. K., Banchereau J., Abrams J. S. Development of polyclonal and monoclonal antibodies for immunoassay and neutralization of human interleukin-4. J Immunol Methods. 1989 Feb 8;117(1):67–81. doi: 10.1016/0022-1759(89)90120-8. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles S., Cunningham-Rundles C., Siegal F. P., Gupta S., Smithwick E. M., Kosloff C., Good R. A. Defective cellular immune response in vitro in common variable immunodeficiency. J Clin Immunol. 1981 Jan;1(1):65–72. doi: 10.1007/BF00915478. [DOI] [PubMed] [Google Scholar]
- Defrance T., Aubry J. P., Vanbervliet B., Banchereau J. Human interferon-gamma acts as a B cell growth factor in the anti-IgM antibody co-stimulatory assay but has no direct B cell differentiation activity. J Immunol. 1986 Dec 15;137(12):3861–3867. [PubMed] [Google Scholar]
- Defrance T., Vanbervliet B., Aubry J. P., Takebe Y., Arai N., Miyajima A., Yokota T., Lee F., Arai K., de Vries J. E. B cell growth-promoting activity of recombinant human interleukin 4. J Immunol. 1987 Aug 15;139(4):1135–1141. [PubMed] [Google Scholar]
- Favre C., Wijdenes J., Cabrillat H., Djossou O., Banchereau J., de Vries J. E. Epitope mapping of recombinant human gamma interferon using monoclonal antibodies. Mol Immunol. 1989 Jan;26(1):17–25. doi: 10.1016/0161-5890(89)90015-1. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Kehrl J. H., Muraguchi A., Goldsmith P. K., Fauci A. S. The direct effects of interleukin 1, interleukin 2, interferon-alpha, interferon-gamma, B-cell growth factor, and a B-cell differentiation factor on resting and activated human B cells. Cell Immunol. 1985 Nov;96(1):38–48. doi: 10.1016/0008-8749(85)90338-7. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. B-cell stimulatory factors (BSFs): molecular structure, biological function, and regulation of expression. J Clin Immunol. 1987 Sep;7(5):343–355. doi: 10.1007/BF00917012. [DOI] [PubMed] [Google Scholar]
- Kruger G., Welte K., Ciobanu N., Cunningham-Rundles C., Ralph P., Venuta S., Feldman S., Koziner B., Wang C. Y., Moore M. A. Interleukin-2 correction of defective in vitro T-cell mitogenesis in patients with common varied immunodeficiency. J Clin Immunol. 1984 Jul;4(4):295–303. doi: 10.1007/BF00915297. [DOI] [PubMed] [Google Scholar]
- Leibson H. J., Gefter M., Zlotnik A., Marrack P., Kappler J. W. Role of gamma-interferon in antibody-producing responses. 1984 Jun 28-Jul 4Nature. 309(5971):799–801. doi: 10.1038/309799a0. [DOI] [PubMed] [Google Scholar]
- Lê thi Bich-Thuy, Fauci A. S. Recombinant interleukin 2 and gamma-interferon act synergistically on distinct steps of in vitro terminal human B cell maturation. J Clin Invest. 1986 Apr;77(4):1173–1179. doi: 10.1172/JCI112418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Botet M., Fontán G., Garcia Rodriguez M. C., de Landázuri M. O. Relationship between IL 2 synthesis and the proliferative response to PHA in different primary immunodeficiencies. J Immunol. 1982 Feb;128(2):679–683. [PubMed] [Google Scholar]
- Matheson D. S., Green B. J. Defect in production of B cell differentiation factor-like activity by mononuclear cells from a boy with hypogammaglobulinemia. J Immunol. 1987 Apr 15;138(8):2469–2472. [PubMed] [Google Scholar]
- Matricardi P. M., Capobianchi M. R., Paganelli R., Facchini J., Sirianni M. C., Seminara R., Dianzani F., Aiuti F. Interferon production in primary immunodeficiencies. J Clin Immunol. 1984 Sep;4(5):388–394. doi: 10.1007/BF00917142. [DOI] [PubMed] [Google Scholar]
- Mayer L., Fu S. M., Cunningham-Rundles C., Kunkel H. G. Polyclonal immunoglobulin secretion in patients with common variable immunodeficiency using monoclonal B cell differentiation factors. J Clin Invest. 1984 Dec;74(6):2115–2120. doi: 10.1172/JCI111636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki T., Suzuki T., Butler J. L., Cooper M. D. Interleukin-2 effects on human B cells activated in vivo. J Clin Immunol. 1987 Jul;7(4):277–287. doi: 10.1007/BF00915548. [DOI] [PubMed] [Google Scholar]
- Muraguchi A., Hirano T., Tang B., Matsuda T., Horii Y., Nakajima K., Kishimoto T. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med. 1988 Feb 1;167(2):332–344. doi: 10.1084/jem.167.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Nakagawa N., Goldstein H., Volkman D. J., Fauci A. S. Demonstration that human B cells respond differently to interleukin 2 and B cell differentiation factor based on their stages of maturation. J Immunol. 1986 Nov 15;137(10):3175–3182. [PubMed] [Google Scholar]
- Paliard X., de Waal Malefijt R., Yssel H., Blanchard D., Chrétien I., Abrams J., de Vries J., Spits H. Simultaneous production of IL-2, IL-4, and IFN-gamma by activated human CD4+ and CD8+ T cell clones. J Immunol. 1988 Aug 1;141(3):849–855. [PubMed] [Google Scholar]
- Perri R. T., Weisdorf D. J. Impaired responsiveness to B cell growth factor in a patient with common variable hypogammaglobulinemia. Blood. 1985 Aug;66(2):345–349. [PubMed] [Google Scholar]
- Pène J., Rousset F., Brière F., Chrétien I., Bonnefoy J. Y., Spits H., Yokota T., Arai N., Arai K., Banchereau J. IgE production by normal human lymphocytes is induced by interleukin 4 and suppressed by interferons gamma and alpha and prostaglandin E2. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6880–6884. doi: 10.1073/pnas.85.18.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Geha R., Wohl M. E., Morimoto C., Rosen F. S., Schlossman S. F. Immunodeficiency associated with loss of T4+ inducer T-cell function. N Engl J Med. 1981 Apr 2;304(14):811–816. doi: 10.1056/NEJM198104023041403. [DOI] [PubMed] [Google Scholar]
- Robin P. E., Shortridge R. T. Lateralisation of tumours of the nasal cavity and paranasal sinuses and its relationship to aetiology. Lancet. 1979 Mar 31;1(8118):695–696. doi: 10.1016/s0140-6736(79)91148-6. [DOI] [PubMed] [Google Scholar]
- Romagnani S., Giudizi M. G., Biagiotti R., Almerigogna F., Mingari C., Maggi E., Liang C. M., Moretta L. B cell growth factor activity of interferon-gamma. Recombinant human interferon-gamma promotes proliferation of anti-mu-activated human B lymphocytes. J Immunol. 1986 May 15;136(10):3513–3516. [PubMed] [Google Scholar]
- Rosen F. S., Cooper M. D., Wedgwood R. J. The primary immunodeficiencies. (2). N Engl J Med. 1984 Aug 2;311(5):300–310. doi: 10.1056/NEJM198408023110506. [DOI] [PubMed] [Google Scholar]
- Saiki O., Ralph P., Cunningham-Rundles C., Good R. A. Three distinct stages of B-cell defects in common varied immunodeficiency. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6008–6012. doi: 10.1073/pnas.79.19.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki O., Shimizu M., Saeki Y., Kishimoto S., Kishimoto T. Dissociation in the production of B cell-stimulating factors (BCGF and BCDF) and interleukin 2 by T cells from a common variable immunodeficient patient. J Immunol. 1984 Oct;133(4):1920–1924. [PubMed] [Google Scholar]
- Sidman C. L., Marshall J. D., Shultz L. D., Gray P. W., Johnson H. M. Gamma-interferon is one of several direct B cell-maturing lymphokines. 1984 Jun 28-Jul 4Nature. 309(5971):801–804. doi: 10.1038/309801a0. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Spits H., Yssel H., Takebe Y., Arai N., Yokota T., Lee F., Arai K., Banchereau J., de Vries J. E. Recombinant interleukin 4 promotes the growth of human T cells. J Immunol. 1987 Aug 15;139(4):1142–1147. [PubMed] [Google Scholar]


