Abstract
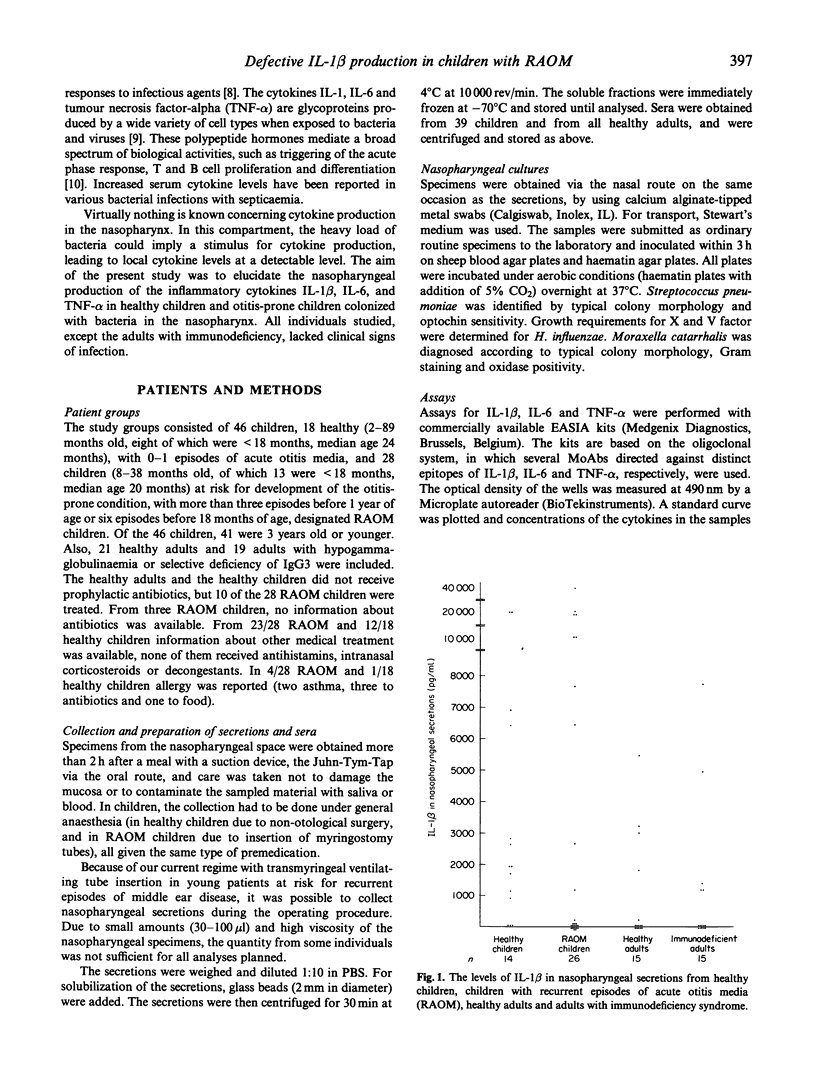
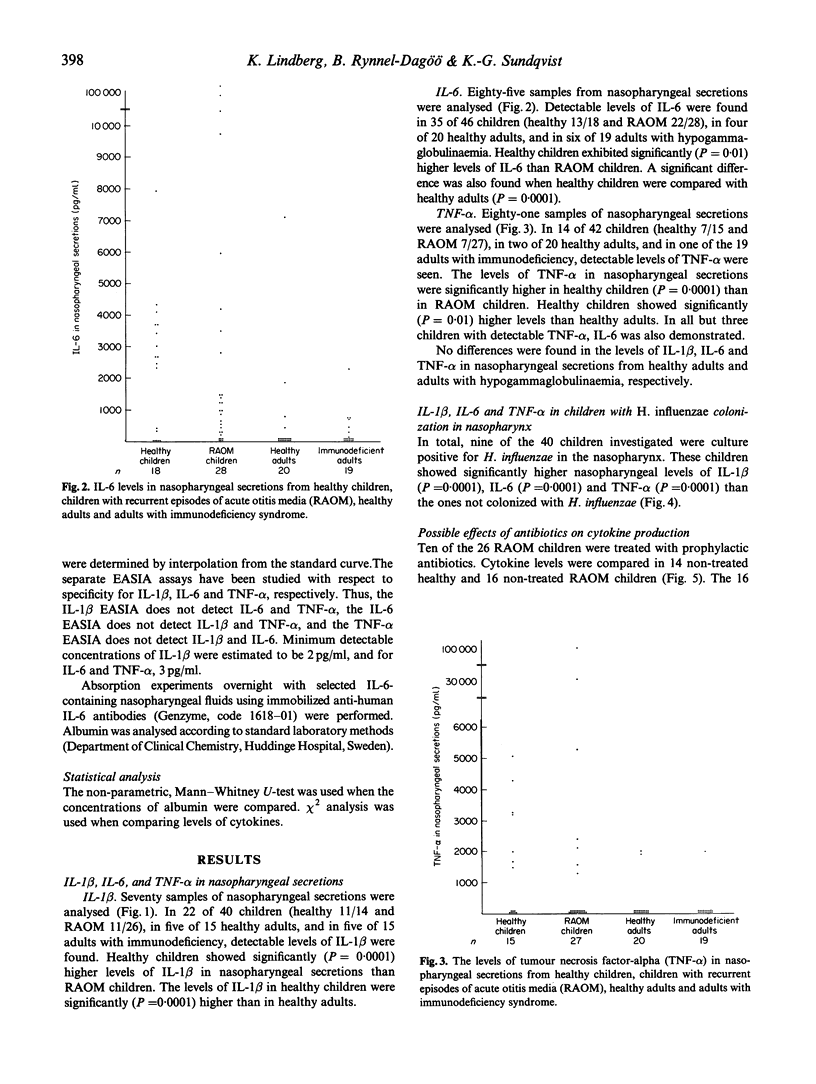
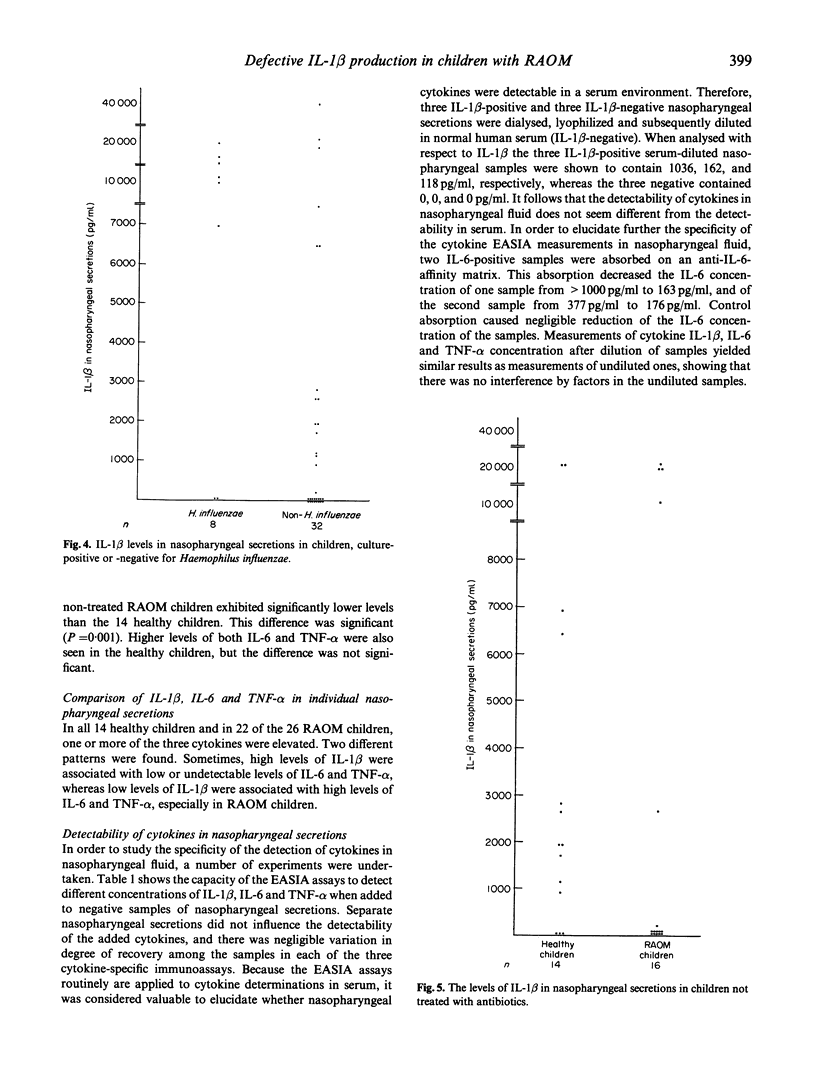
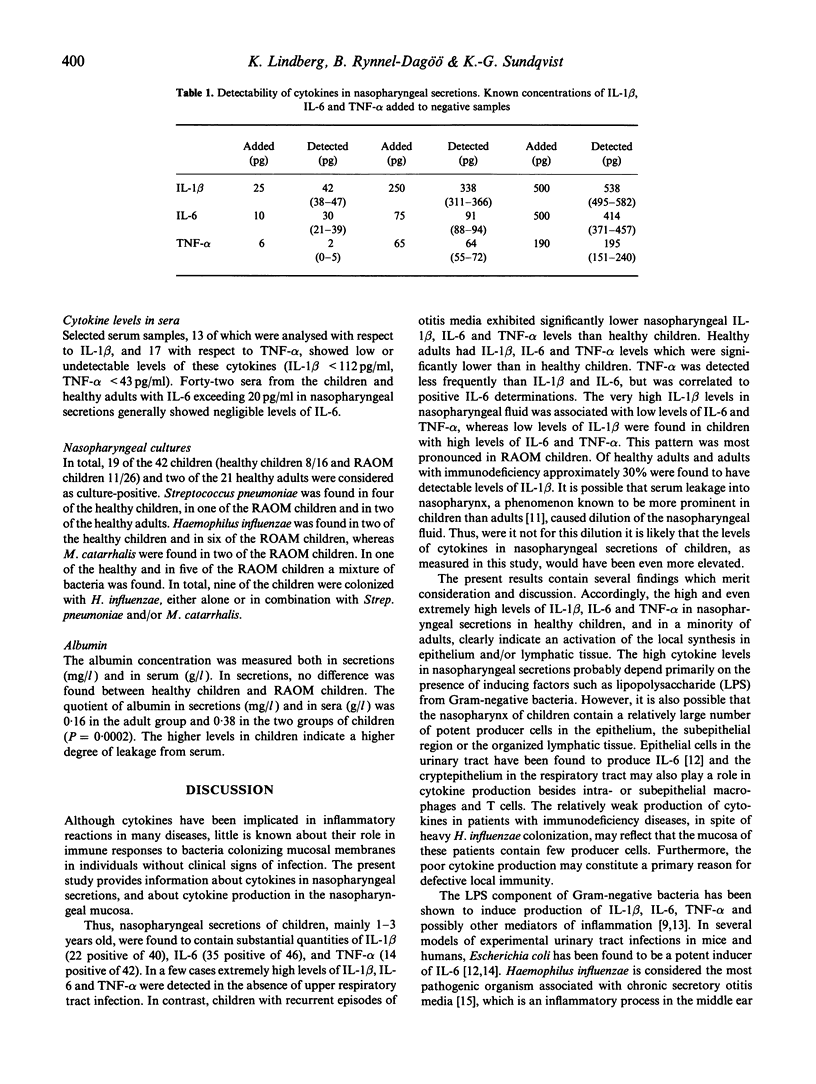
The host-parasite relationship in the nasopharynx of young children with bacterial colonization and antigen uptake in the mucosa and lymphatic tissue provides an opportunity to investigate infectious/inflammatory processes and responses. IL-1 beta, IL-6 and tumour necrosis factor-alpha (TNF-alpha) were analysed in nasopharyngeal secretions and serum from children with or without recurrent episodes of acute otitis media, from healthy adults and adults with or without recurrent episodes of acute otitis media, from healthy adults and adults with hypogammaglobulinaemia or selective deficiency of IgG3. Nasopharyngeal secretions generally contained substantial amounts of IL-1 beta, IL-6 and TNF-alpha. In contrast, IL-1 beta, IL-6 and TNF-alpha were not detectable in sera on the same occasion. Children were found to have higher levels of IL-1 beta, IL-6 and TNF-alpha than healthy adults and than adults with immunodeficiency. High levels of IL-1 beta were associated with low or undetectable levels of IL-6 and TNF-alpha, whereas the opposite pattern was seen in association with low levels of IL-1 beta. This was especially true for children with recurrent episodes of acute otitis media (RAOM). In children with nasopharyngeal colonization with Haemophilus influenzae, significantly higher levels of IL-1 beta, IL-6 and TNF-alpha (P = 0.0001, respectively) were found compared with non-colonized children. Notably, the RAOM children exhibited significantly lower levels of IL-1 beta, IL-6, and TNF-alpha in nasopharyngeal secretions (P = 0.0001, 0.01 and 0.0001, respectively) than healthy children. These results demonstrate local production of inflammatory cytokines in nasopharynx, related to bacterial colonization, and suggest that children with RAOM are poor nasopharyngeal cytokine producers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Kishimoto T. IL-6 and NF-IL6 in acute-phase response and viral infection. Immunol Rev. 1992 Jun;127:25–50. doi: 10.1111/j.1600-065x.1992.tb01407.x. [DOI] [PubMed] [Google Scholar]
- Andersson B., Eriksson B., Falsen E., Fogh A., Hanson L. A., Nylén O., Peterson H., Svanborg Edén C. Adhesion of Streptococcus pneumoniae to human pharyngeal epithelial cells in vitro: differences in adhesive capacity among strains isolated from subjects with otitis media, septicemia, or meningitis or from healthy carriers. Infect Immun. 1981 Apr;32(1):311–317. doi: 10.1128/iai.32.1.311-317.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F. R., Burke F. The cytokine network. Immunol Today. 1989 Sep;10(9):299–304. doi: 10.1016/0167-5699(89)90085-6. [DOI] [PubMed] [Google Scholar]
- DeMaria T. F., Prior R. B., Briggs B. R., Lim D. J., Birck H. G. Endotoxin in middle-ear effusions from patients with chronic otitis media with effusion. J Clin Microbiol. 1984 Jul;20(1):15–17. doi: 10.1128/jcm.20.1.15-17.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren J., Samuelson A., Ahlin A., Jonasson J., Rynnel-Dagö B., Lindberg A. Haemophilus influenzae resides and multiplies intracellularly in human adenoid tissue as demonstrated by in situ hybridization and bacterial viability assay. Infect Immun. 1994 Feb;62(2):673–679. doi: 10.1128/iai.62.2.673-679.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren J., Samuelson A., Lindberg A., Rynnel-Dagö B. Quantitative bacterial culture from adenoid lymphatic tissue with special reference to Haemophilus [corrected]. Acta Otolaryngol. 1993 Sep;113(5):668–672. doi: 10.3109/00016489309135882. [DOI] [PubMed] [Google Scholar]
- Freijd A., Bygdeman S., Rynnel-Dagö B. The nasopharyngeal microflora of otitis-prone children, with emphasis on H. influenzae. Acta Otolaryngol. 1984 Jan-Feb;97(1-2):117–126. doi: 10.3109/00016488409130971. [DOI] [PubMed] [Google Scholar]
- Freijd A., Oxelius V. A., Rynnel-Dagö B. A prospective study demonstrating an association between plasma IgG2 concentrations and susceptibility to otitis media in children. Scand J Infect Dis. 1985;17(1):115–120. doi: 10.3109/00365548509070430. [DOI] [PubMed] [Google Scholar]
- Gray B. M., Converse G. M., 3rd, Dillon H. C., Jr Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980 Dec;142(6):923–933. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- Harsten G., Prellner K., Heldrup J., Kalm O., Kornfält R. Recurrent acute otitis media. A prospective study of children during the first three years of life. Acta Otolaryngol. 1989 Jan-Feb;107(1-2):111–119. doi: 10.3109/00016488909127487. [DOI] [PubMed] [Google Scholar]
- Heney D., Lewis I. J., Evans S. W., Banks R., Bailey C. C., Whicher J. T. Interleukin-6 and its relationship to C-reactive protein and fever in children with febrile neutropenia. J Infect Dis. 1992 May;165(5):886–890. doi: 10.1093/infdis/165.5.886. [DOI] [PubMed] [Google Scholar]
- Kamme C., Lundgren K. Frequency of typable and non-typable Haemophilus influenzae strains in children with acute otitis media and results of penicillin V treatment. Scand J Infect Dis. 1971;3(3):225–228. doi: 10.3109/inf.1971.3.issue-3.08. [DOI] [PubMed] [Google Scholar]
- Kawano M., Hirano T., Matsuda T., Taga T., Horii Y., Iwato K., Asaoku H., Tang B., Tanabe O., Tanaka H. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988 Mar 3;332(6159):83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- Lim D. J., DeMaria T. F. Panel discussion: pathogenesis of otitis media. Bacteriology and immunology. Laryngoscope. 1982 Mar;92(3):278–286. doi: 10.1288/00005537-198203000-00011. [DOI] [PubMed] [Google Scholar]
- Lindberg K., Freijd A., Rynnel-Dagö B., Hammarström L. Anti pneumococcal antibody activity in nasopharyngeal secretions in healthy adults and children. Acta Otolaryngol. 1993 Sep;113(5):673–678. doi: 10.3109/00016489309135883. [DOI] [PubMed] [Google Scholar]
- Loos B. G., Bernstein J. M., Dryja D. M., Murphy T. F., Dickinson D. P. Determination of the epidemiology and transmission of nontypable Haemophilus influenzae in children with otitis media by comparison of total genomic DNA restriction fingerprints. Infect Immun. 1989 Sep;57(9):2751–2757. doi: 10.1128/iai.57.9.2751-2757.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prellner K., Kalm O., Harsten G., Heldrup J., Oxelius V. A. Pneumococcal serum antibody concentrations during the first three years of life: a study of otitis-prone and non-otitis-prone children. Int J Pediatr Otorhinolaryngol. 1989 Jul;17(3):267–279. doi: 10.1016/0165-5876(89)90052-9. [DOI] [PubMed] [Google Scholar]
- Pullicino E. A., Carli F., Poole S., Rafferty B., Malik S. T., Elia M. The relationship between the circulating concentrations of interleukin 6 (IL-6), tumor necrosis factor (TNF) and the acute phase response to elective surgery and accidental injury. Lymphokine Res. 1990 Summer;9(2):231–238. [PubMed] [Google Scholar]
- Riesenfeld-Orn I., Wolpe S., Garcia-Bustos J. F., Hoffmann M. K., Tuomanen E. Production of interleukin-1 but not tumor necrosis factor by human monocytes stimulated with pneumococcal cell surface components. Infect Immun. 1989 Jul;57(7):1890–1893. doi: 10.1128/iai.57.7.1890-1893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J. S., Kilpatrick L., Costarino A. T., Jr, Lee S. C., Harris M. C. Correlation of plasma cytokine elevations with mortality rate in children with sepsis. J Pediatr. 1992 Apr;120(4 Pt 1):510–515. doi: 10.1016/s0022-3476(05)82476-x. [DOI] [PubMed] [Google Scholar]
- Trottier S., Stenberg K., Svanborg-Edén C. Turnover of nontypable Haemophilus influenzae in the nasopharynges of healthy children. J Clin Microbiol. 1989 Oct;27(10):2175–2179. doi: 10.1128/jcm.27.10.2175-2179.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- Waage A., Halstensen A., Shalaby R., Brandtzaeg P., Kierulf P., Espevik T. Local production of tumor necrosis factor alpha, interleukin 1, and interleukin 6 in meningococcal meningitis. Relation to the inflammatory response. J Exp Med. 1989 Dec 1;170(6):1859–1867. doi: 10.1084/jem.170.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. G., Clark S. C. Multiple actions of interleukin 6 within a cytokine network. Immunol Today. 1988 May;9(5):137–139. doi: 10.1016/0167-5699(88)91200-5. [DOI] [PubMed] [Google Scholar]
- Yellon R. F., Leonard G., Marucha P. T., Craven R., Carpenter R. J., Lehmann W. B., Burleson J. A., Kreutzer D. L. Characterization of cytokines present in middle ear effusions. Laryngoscope. 1991 Feb;101(2):165–169. doi: 10.1288/00005537-199102000-00011. [DOI] [PubMed] [Google Scholar]
- de Man P., van Kooten C., Aarden L., Engberg I., Linder H., Svanborg Edén C. Interleukin-6 induced at mucosal surfaces by gram-negative bacterial infection. Infect Immun. 1989 Nov;57(11):3383–3388. doi: 10.1128/iai.57.11.3383-3388.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


