Abstract
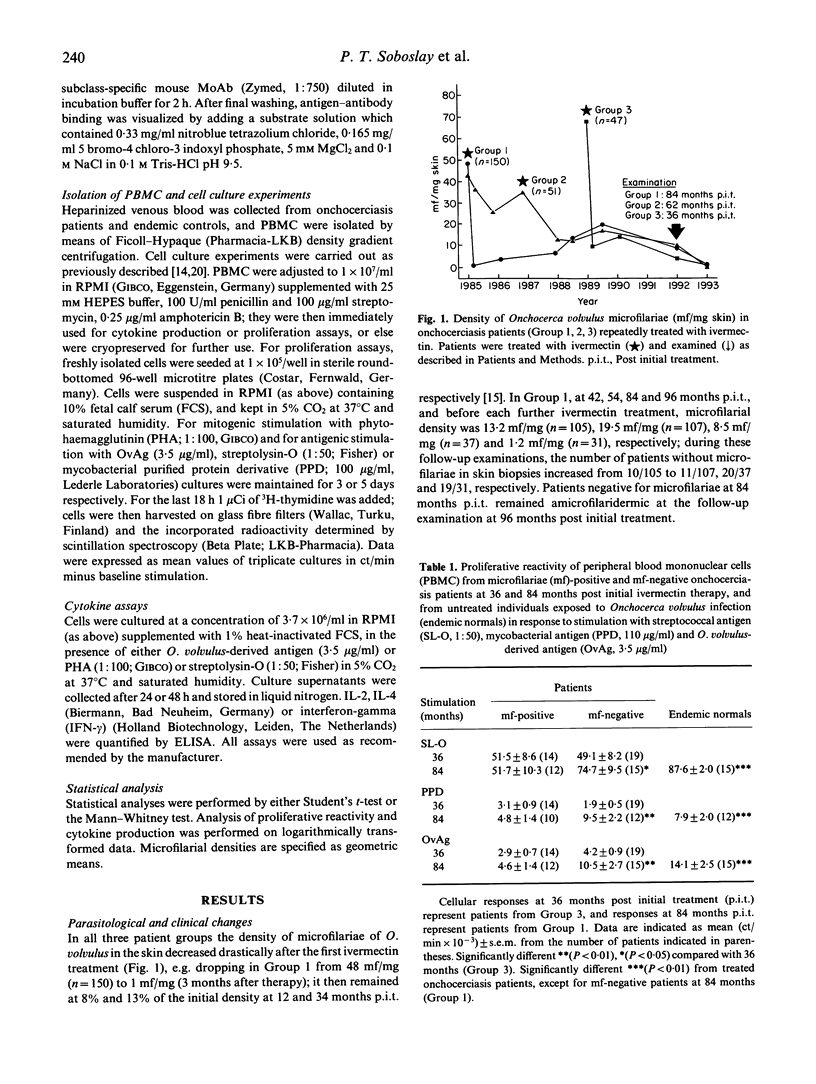
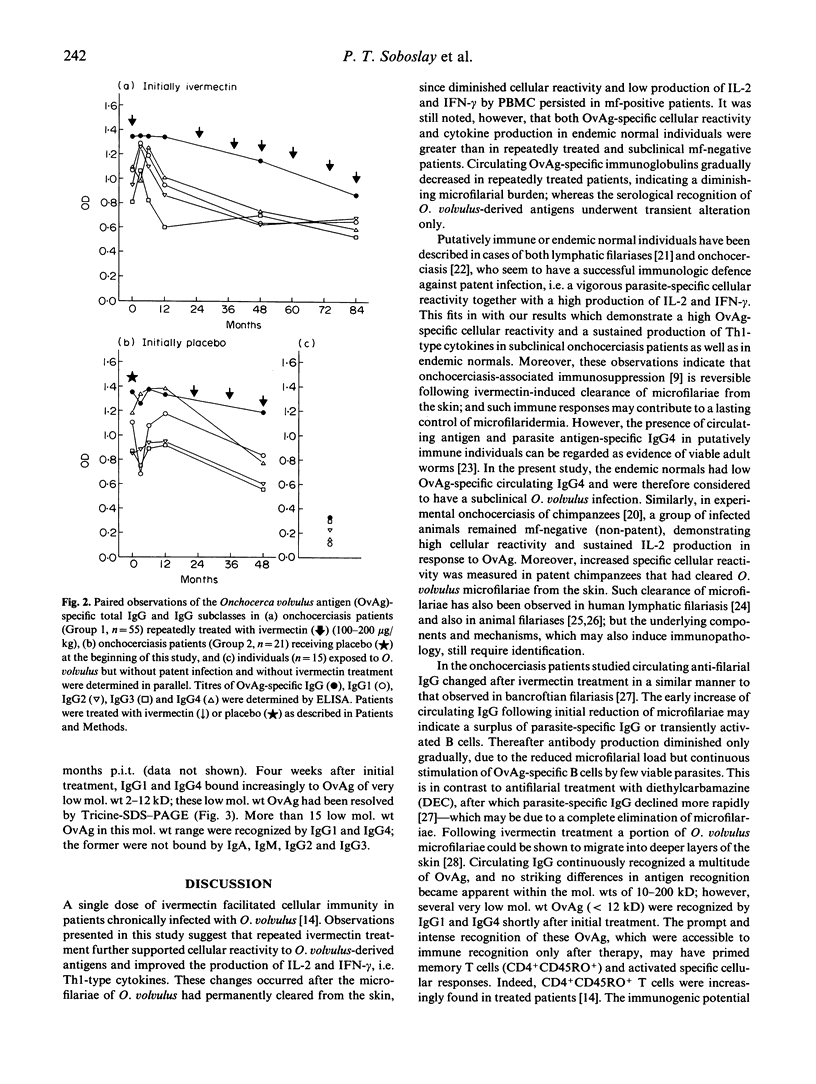
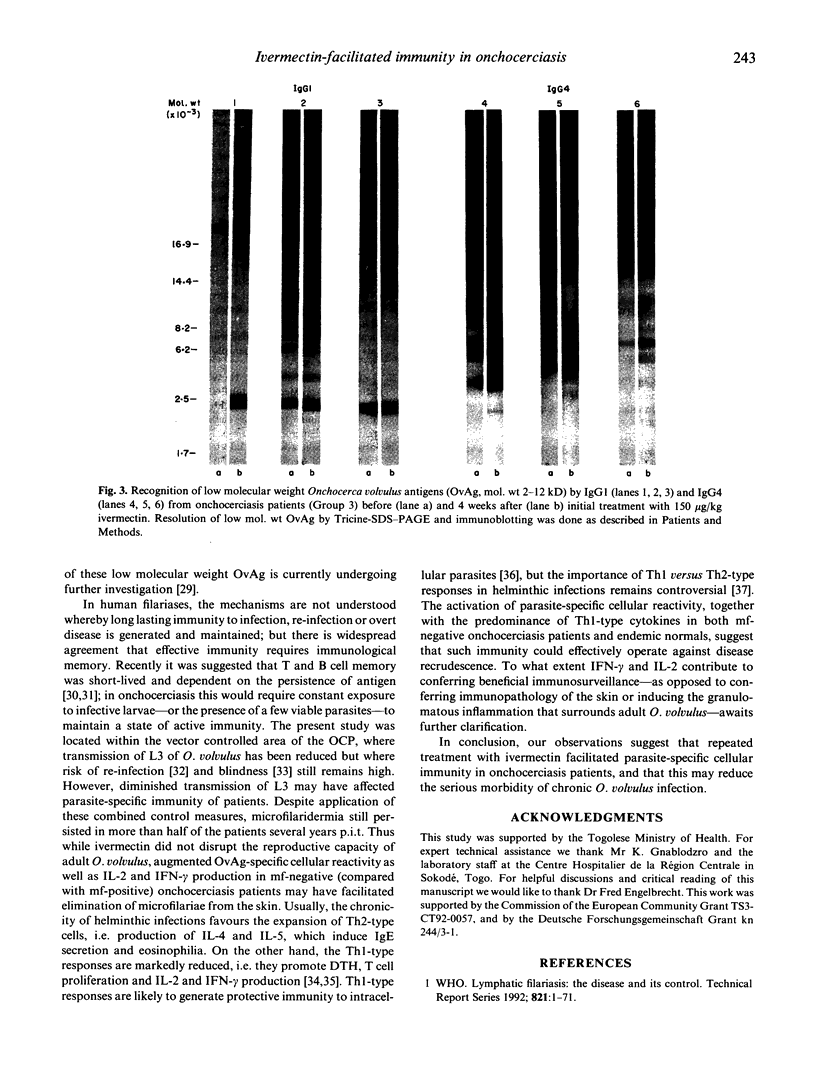
The present study examined the quantitative and qualitative changes registered in the parasite-specific antibody response, cellular reactivity and cytokine production profile in onchocerciasis patients repeatedly treated with ivermectin over a period of 8 years. The densities of Onchocerca volvulus microfilariae (mf) in treated patients remained significantly reduced, whereas the number of permanently amicrofilaridermic patients (subclinical infection) increased with repeated treatments. In vitro cellular responses to O. volvulus antigen (OvAg) were highest (P<0.01) in untreated control individuals exposed to infection, but negative for mf of O. volvulus (endemic normals). Cellular reactivity in repeatedly treated patients was higher at 84 than at 36 months post initial treatment (p.i.t.); furthermore, the proliferative responses to OvAg, mycobacterial purified protein derivative (PPD) and streptococcal SL-O were greater (P<0.05) at 84 months p.i.t. in amicrofilaridermic than in microfilaria-positive onchocerciasis patients. In amicrofilaridermic patients such reactivity approached the magnitude observed in endemic normals. Peripheral blood mononuclear cells (PBMC) from patients and endemic normals produced equivalent amounts of IL-2, IL-4 and interferon-gamma (IFN-γ) in response to mitogenic stimulation with phytohaemagglutinin (PHA); in response to OvAg, however, significantly more IL-2 and IFN-γ were produced by PBMC from subclinical amicrofilaridermic patients or endemic normals than by mf-positive patients. OvAg-specific production of IL-4 by PBMC from treated patients was lower at 84 than at 36 months p.i.t. At three months p.i.t. the titres of circulating OvAg-specific IgG1-3 had increased (P<0.05), but they then continuously declined with repeated treatments. Only IgG1 and IgG4 bound to OvAg of mol. wt 2-12 kD at 1 month p.i.t., while recognition of OvAg of mol. wt 10-200 kD by IgG1, IgG2 and IgG4 reached a maximum intensity at 3-6 months p.i.t., with the overall intensity of binding to OvAg gradually weakening thereafter. These results suggest that onchocerciasis-associated immunosuppression is reversible following ivermectin-induced permanent clearance of microfilariae from the skin; and that a vigorous parasite-specific cellular reactivity and a sustained production of IL-2 and IFN-γ in amicrofilaridermic individuals may contribute to controlling O. volvulus infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aziz M. A., Diallo S., Diop I. M., Lariviere M., Porta M. Efficacy and tolerance of ivermectin in human onchocerciasis. Lancet. 1982 Jul 24;2(8291):171–173. doi: 10.1016/s0140-6736(82)91026-1. [DOI] [PubMed] [Google Scholar]
- Dafa'alla T. H., Ghalib H. W., Abdelmageed A., Williams J. F. The profile of IgG and IgG subclasses of onchocerciasis patients. Clin Exp Immunol. 1992 May;88(2):258–263. doi: 10.1111/j.1365-2249.1992.tb03070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day K. P. The endemic normal in lymphatic filariasis: A static concept. Parasitol Today. 1991 Dec;7(12):341–343. doi: 10.1016/0169-4758(91)90215-a. [DOI] [PubMed] [Google Scholar]
- De Sole G., Accorsi S., Cresveaux H., Remme J., Walsh F., Hendrickx J. Distribution and severity of onchocerciasis in southern Benin, Ghana and Togo. Acta Trop. 1992 Dec;52(2-3):87–97. doi: 10.1016/0001-706x(92)90024-r. [DOI] [PubMed] [Google Scholar]
- De Sole G., Remme J. Onchocerciasis infection in children born during 14 years of Simulium control in West Africa. Trans R Soc Trop Med Hyg. 1991 May-Jun;85(3):385–390. doi: 10.1016/0035-9203(91)90301-e. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Pearce E. J., Urban J. F., Jr, Sher A. Regulation and biological function of helminth-induced cytokine responses. Immunol Today. 1991 Mar;12(3):A62–A66. doi: 10.1016/S0167-5699(05)80018-0. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Urban J. F., Jr Cytokines: making the right choice. Parasitol Today. 1992 Sep;8(9):311–314. doi: 10.1016/0169-4758(92)90105-b. [DOI] [PubMed] [Google Scholar]
- Freedman D. O., Nutman T. B., Ottesen E. A. Protective immunity in bancroftian filariasis. Selective recognition of a 43-kD larval stage antigen by infection-free individuals in an endemic area. J Clin Invest. 1989 Jan;83(1):14–22. doi: 10.1172/JCI113850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin M., Edmonds K., Ellner J. J., Erttmann K. D., White A. T., Newland H. S., Taylor H. R., Greene B. M. Cell-mediated immune responses in human infection with Onchocerca volvulus. J Immunol. 1988 Mar 15;140(6):1999–2007. [PubMed] [Google Scholar]
- Gray D., Matzinger P. T cell memory is short-lived in the absence of antigen. J Exp Med. 1991 Nov 1;174(5):969–974. doi: 10.1084/jem.174.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D., Skarvall H. B-cell memory is short-lived in the absence of antigen. Nature. 1988 Nov 3;336(6194):70–73. doi: 10.1038/336070a0. [DOI] [PubMed] [Google Scholar]
- Greene B. M., Fanning M. M., Ellner J. J. Non-specific suppression of antigen-induced lymphocyte blastogenesis in Onchocerca volvulus infection in man. Clin Exp Immunol. 1983 May;52(2):259–265. [PMC free article] [PubMed] [Google Scholar]
- Greene B. M. Modern medicine versus an ancient scourge: progress toward control of onchocerciasis. J Infect Dis. 1992 Jul;166(1):15–21. doi: 10.1093/infdis/166.1.15. [DOI] [PubMed] [Google Scholar]
- Jürgens S., Schulz-Key H. Effect of ivermectin on the vertical distribution of Onchocerca volvulus microfilariae in the skin. Trop Med Parasitol. 1990 Jun;41(2):165–168. [PubMed] [Google Scholar]
- Karam M., Weiss N. Seroepidemiological investigations of onchocerciasis in a hyperendemic area of West Africa. Am J Trop Med Hyg. 1985 Sep;34(5):907–917. doi: 10.4269/ajtmh.1985.34.907. [DOI] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Lüder C. G., Soboslay P. T., Prince A. M., Greene B. M., Lucius R., Schulz-Key H. Experimental onchocerciasis in chimpanzees: cellular responses and antigen recognition after immunization and challenge with Onchocerca volvulus infective third-stage larvae. Parasitology. 1993 Jul;107(Pt 1):87–97. doi: 10.1017/s0031182000079440. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Nutman T. B., Steel C., Ward D. J., Zea-Flores G., Ottesen E. A. Immunity to onchocerciasis: recognition of larval antigens by humans putatively immune to Onchocerca volvulus infection. J Infect Dis. 1991 May;163(5):1128–1133. doi: 10.1093/infdis/163.5.1128. [DOI] [PubMed] [Google Scholar]
- Ottesen E. A. Immunological aspects of lymphatic filariasis and onchocerciasis in man. Trans R Soc Trop Med Hyg. 1984;78 (Suppl):9–18. doi: 10.1016/0035-9203(84)90309-2. [DOI] [PubMed] [Google Scholar]
- Remme J., De Sole G., Dadzie K. Y., Alley E. S., Baker R. H., Habbema J. D., Plaisier A. P., van Oortmarssen G. J., Samba E. M. Large scale ivermectin distribution and its epidemiological consequences. Acta Leiden. 1990;59(1-2):177–191. [PubMed] [Google Scholar]
- Remme J., De Sole G., van Oortmarssen G. J. The predicted and observed decline in onchocerciasis infection during 14 years of successful control of Simulium spp. in west Africa. Bull World Health Organ. 1990;68(3):331–339. doi: 10.1089/ten.2005.11.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz-Key H., Albiez E. J., Büttner D. W. Isolation of living adult Onchocerca volvulus from nodules. Tropenmed Parasitol. 1977 Dec;28(4):428–430. [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Soboslay P. T., Dreweck C. M., Hoffmann W. H., Lüder C. G., Heuschkel C., Görgen H., Banla M., Schulz-Key H. Ivermectin-facilitated immunity in onchocerciasis. Reversal of lymphocytopenia, cellular anergy and deficient cytokine production after single treatment. Clin Exp Immunol. 1992 Sep;89(3):407–413. doi: 10.1111/j.1365-2249.1992.tb06971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboslay P. T., Dreweck C. M., Taylor H. R., Brotman B., Wenk P., Greene B. M. Experimental onchocerciasis in chimpanzees. Cell-mediated immune responses, and production and effects of IL-1 and IL-2 with Onchocerca volvulus infection. J Immunol. 1991 Jul 1;147(1):346–353. [PubMed] [Google Scholar]
- Steel C., Lujan-Trangay A., Gonzalez-Peralta C., Zea-Flores G., Nutman T. B. Immunologic responses to repeated ivermectin treatment in patients with onchocerciasis. J Infect Dis. 1991 Sep;164(3):581–587. doi: 10.1093/infdis/164.3.581. [DOI] [PubMed] [Google Scholar]
- Urban J. F., Jr, Madden K. B., Svetić A., Cheever A., Trotta P. P., Gause W. C., Katona I. M., Finkelman F. D. The importance of Th2 cytokines in protective immunity to nematodes. Immunol Rev. 1992 Jun;127:205–220. doi: 10.1111/j.1600-065x.1992.tb01415.x. [DOI] [PubMed] [Google Scholar]
- Wamae C. N., Roberts J. M., Eberhard M. L., Lammie P. J. Kinetics of circulating human IgG4 after diethylcarbamazine and ivermectin treatment of bancroftian filariasis. J Infect Dis. 1992 Jun;165(6):1158–1160. doi: 10.1093/infdis/165.6.1158. [DOI] [PubMed] [Google Scholar]
- Ward D. J., Nutman T. B., Zea-Flores G., Portocarrero C., Lujan A., Ottesen E. A. Onchocerciasis and immunity in humans: enhanced T cell responsiveness to parasite antigen in putatively immune individuals. J Infect Dis. 1988 Mar;157(3):536–543. doi: 10.1093/infdis/157.3.536. [DOI] [PubMed] [Google Scholar]



