Abstract
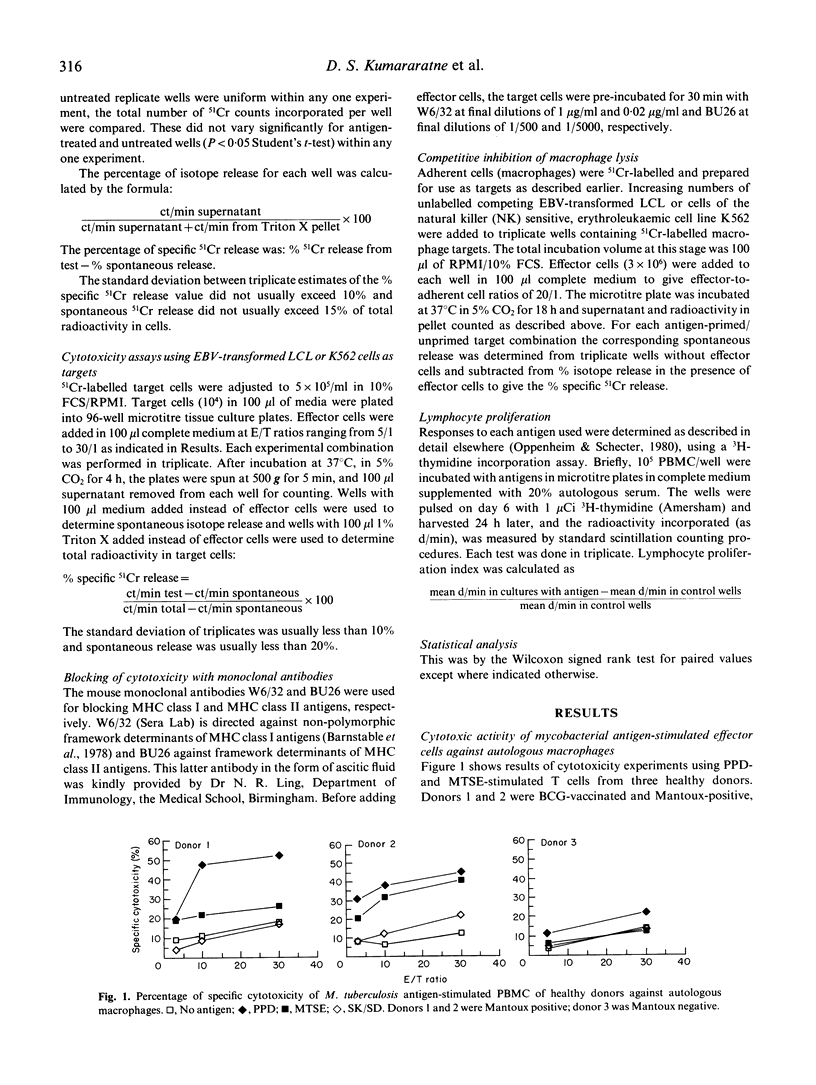
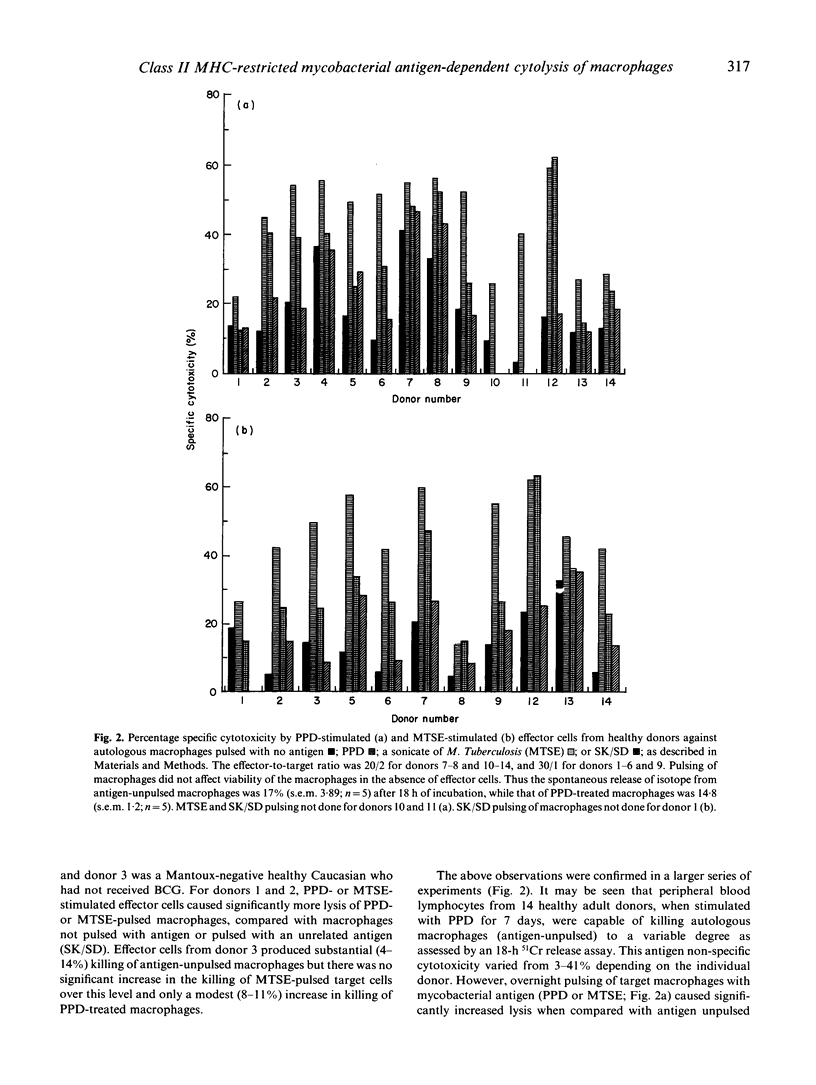
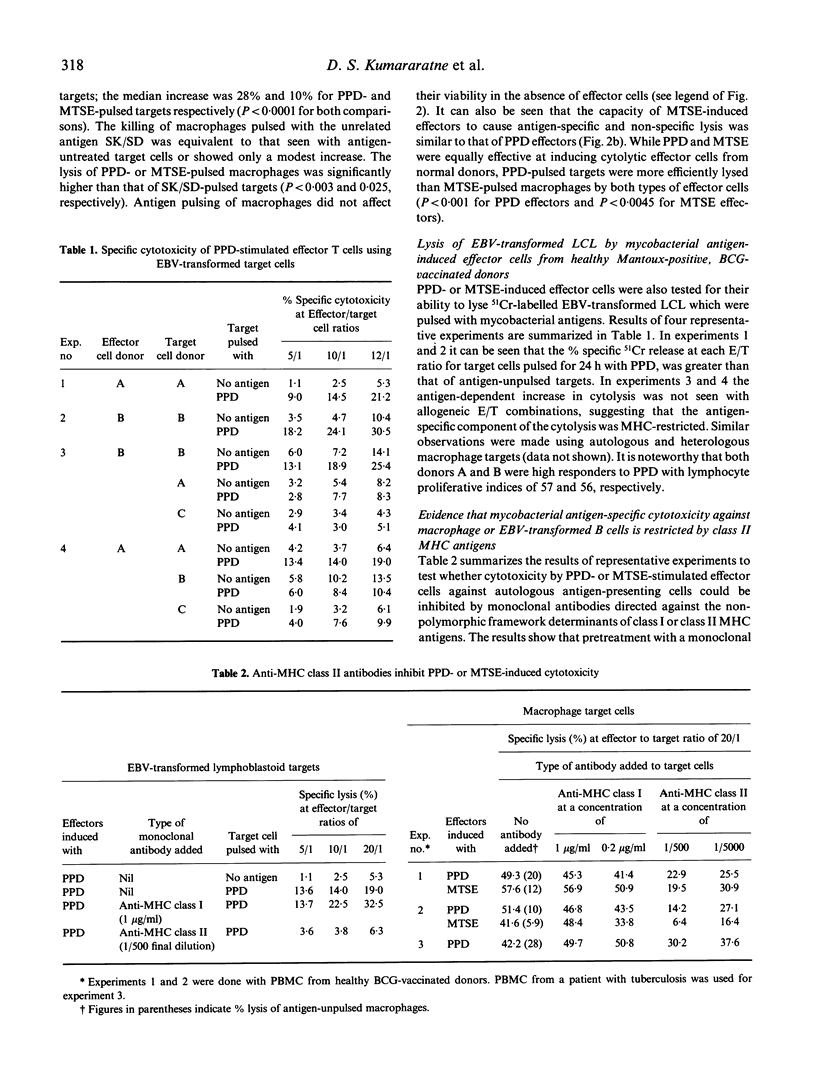
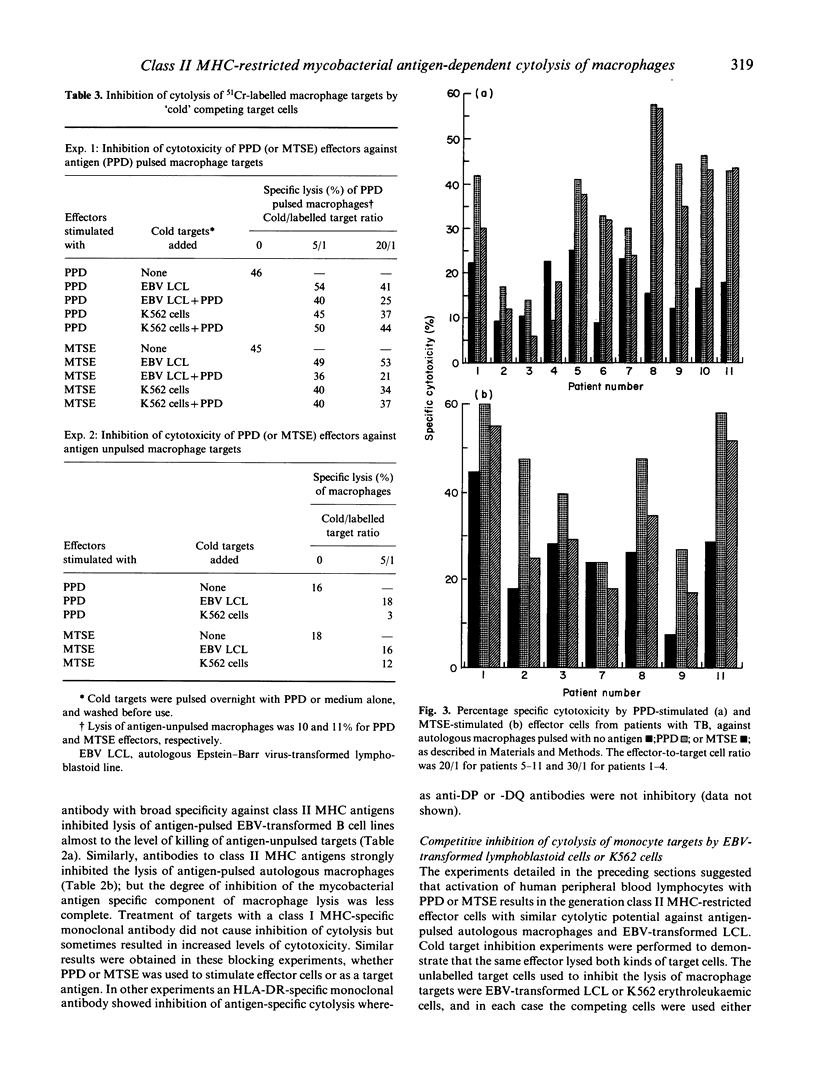
The cytolytic capacity of mycobacterial antigen-stimulated peripheral blood mononuclear cells, from healthy Mantoux-positive volunteers and from patients with tuberculosis was investigated. Polyclonal T cell lines induced by 7 days of stimulation in vitro with PPD or a sonicate of Mycobacterium tuberculosis lysed both autologous macrophages and Epstein-Barr virus (EBV) transformed B cell lines which had been pulsed with mycobacterial antigens, to a greater extent than unpulsed target cells or target cells pulsed with an irrelevant antigen (streptokinase/streptodornase). The killing of mycobacterial antigen-pulsed macrophages and EBV-transformed B cell line targets was inhibited by monoclonal antibodies to MHC class II antigens but not by antibodies directed against MHC class I antigens. PPD-pulsed EBV-transformed lymphoblastoid cell lines (LCL) competitively inhibited the killing of mycobacterial antigen-pulsed macrophages, whereas natural killer (NK) sensitive K562 cells (with or without antigen pulsing) did not inhibit mycobacterial antigen-dependent cytolysis of macrophages. Patients with tuberculosis showed a spectrum of mycobacterial antigen-induced cytolytic capacity. Those with extensive tissue necrosis (e.g. cavitatory pulmonary tuberculosis or caseous, extrathoracic tuberculosis) had high levels while patients with disseminated (miliary) tuberculosis or disease refractory to treatment showed little evidence of mycobacterial antigen induced cytotoxicity. The ability of mycobacterial antigen-stimulated lymphoblasts to kill specific antigen-pulsed autologous macrophages was not significantly different between healthy donors and patients with tuberculosis. However, the 'mycobacterial antigen-specific' component of this cytolysis was significantly deficient (P less than 0.01) in patients. We conclude that mycobacterial antigen-specific cytotoxic T cell responses may play a significant part in the immune response to mycobacterial infection.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ab B. K., Kiessling R., Van Embden J. D., Thole J. E., Kumararatne D. S., Pisa P., Wondimu A., Ottenhoff T. H. Induction of antigen-specific CD4+ HLA-DR-restricted cytotoxic T lymphocytes as well as nonspecific nonrestricted killer cells by the recombinant mycobacterial 65-kDa heat-shock protein. Eur J Immunol. 1990 Feb;20(2):369–377. doi: 10.1002/eji.1830200221. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Blanchard D. K., Stewart W. E., 2nd, Klein T. W., Friedman H., Djeu J. Y. Cytolytic activity of human peripheral blood leukocytes against Legionella pneumophila-infected monocytes: characterization of the effector cell and augmentation by interleukin 2. J Immunol. 1987 Jul 15;139(2):551–556. [PubMed] [Google Scholar]
- Chiplunkar S., De Libero G., Kaufmann S. H. Mycobacterium leprae-specific Lyt-2+ T lymphocytes with cytolytic activity. Infect Immun. 1986 Dec;54(3):793–797. doi: 10.1128/iai.54.3.793-797.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowle A. J., Sbarbaro J. A., May M. H. Effects of isoniazid and of ceforanide against virulent tubercle bacilli in cultured human macrophages. Tubercle. 1988 Mar;69(1):15–25. doi: 10.1016/0041-3879(88)90036-0. [DOI] [PubMed] [Google Scholar]
- De Libero G., Flesch I., Kaufmann S. H. Mycobacteria-reactive Lyt-2+ T cell lines. Eur J Immunol. 1988 Jan;18(1):59–66. doi: 10.1002/eji.1830180110. [DOI] [PubMed] [Google Scholar]
- Djeu J. Y., Blanchard D. K. Lysis of human monocytes by lymphokine-activated killer cells. Cell Immunol. 1988 Jan;111(1):55–65. doi: 10.1016/0008-8749(88)90050-0. [DOI] [PubMed] [Google Scholar]
- Douvas G. S., Looker D. L., Vatter A. E., Crowle A. J. Gamma interferon activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria. Infect Immun. 1985 Oct;50(1):1–8. doi: 10.1128/iai.50.1.1-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I., Kaufmann S. H. Mycobacterial growth inhibition by interferon-gamma-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J Immunol. 1987 Jun 15;138(12):4408–4413. [PubMed] [Google Scholar]
- Grosset J. Bacteriologic basis of short-course chemotherapy for tuberculosis. Clin Chest Med. 1980 May;1(2):231–241. [PubMed] [Google Scholar]
- Hansen P. W., Kristensen T. Cell mediated PPD specific cytotoxicity against human monocyte targets: III. Cellular typing with CTLs restricted by class II HLA antigens. Tissue Antigens. 1986 Apr;27(4):227–238. doi: 10.1111/j.1399-0039.1986.tb01524.x. [DOI] [PubMed] [Google Scholar]
- Hassan N. F., Campbell D. E., Douglas S. D. Purification of human monocytes on gelatin-coated surfaces. J Immunol Methods. 1986 Dec 24;95(2):273–276. doi: 10.1016/0022-1759(86)90415-1. [DOI] [PubMed] [Google Scholar]
- Hewitt J., Coates A. R., Mitchison D. A., Ivanyi J. The use of murine monoclonal antibodies without purification of antigen in the serodiagnosis of tuberculosis. J Immunol Methods. 1982 Dec 17;55(2):205–211. doi: 10.1016/0022-1759(82)90032-1. [DOI] [PubMed] [Google Scholar]
- Kaplan G., Sheftel G., Job C. K., Mathur N. K., Nath I., Cohn Z. A. Efficacy of a cell-mediated reaction to the purified protein derivative of tuberculin in the disposal of Mycobacterium leprae from human skin. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5210–5214. doi: 10.1073/pnas.85.14.5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. CD8+ T lymphocytes in intracellular microbial infections. Immunol Today. 1988 Jun;9(6):168–174. doi: 10.1016/0167-5699(88)91292-3. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., Väth U., De Libero G. Specific lysis of Listeria monocytogenes-infected macrophages by class II-restricted L3T4+ T cells. Eur J Immunol. 1987 Feb;17(2):237–246. doi: 10.1002/eji.1830170214. [DOI] [PubMed] [Google Scholar]
- Khor M., Lowrie D. B., Coates A. R., Mitchison D. A. Recombinant interferon-gamma and chemotherapy with isoniazid and rifampicin in experimental murine tuberculosis. Br J Exp Pathol. 1986 Aug;67(4):587–596. [PMC free article] [PubMed] [Google Scholar]
- Khor M., Lowrie D. B., Mitchison D. A. Effects of recombinant interferon-gamma and chemotherapy with isoniazid and rifampicin on infections of mouse peritoneal macrophages with Listeria monocytogenes and Mycobacterium microti in vitro. Br J Exp Pathol. 1986 Oct;67(5):707–717. [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A., Bove S. Specific B lymphocytes efficiently pick up, process and present antigen to T cells. Behring Inst Mitt. 1985 Aug;(77):82–87. [PubMed] [Google Scholar]
- Lowrie D. B., Andrew P. W. Macrophage antimycobacterial mechanisms. Br Med Bull. 1988 Jul;44(3):624–634. doi: 10.1093/oxfordjournals.bmb.a072272. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison L. A., Lukacher A. E., Braciale V. L., Fan D. P., Braciale T. J. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med. 1986 Apr 1;163(4):903–921. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa A. S., Godal T. BCG induced CD4+ cytotoxic T cells from BCG vaccinated healthy subjects: relation between cytotoxicity and suppression in vitro. Clin Exp Immunol. 1987 Aug;69(2):255–262. [PMC free article] [PubMed] [Google Scholar]
- Müller I., Cobbold S. P., Waldmann H., Kaufmann S. H. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect Immun. 1987 Sep;55(9):2037–2041. doi: 10.1128/iai.55.9.2037-2041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nencioni L., Villa L., Boraschi D., Berti B., Tagliabue A. Natural and antibody-dependent cell-mediated activity against Salmonella typhimurium by peripheral and intestinal lymphoid cells in mice. J Immunol. 1983 Feb;130(2):903–907. [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Adoptive protection of the Mycobacterium tuberculosis-infected lung. Dissociation between cells that passively transfer protective immunity and those that transfer delayed-type hypersensitivity to tuberculin. Cell Immunol. 1984 Mar;84(1):113–120. doi: 10.1016/0008-8749(84)90082-0. [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., Ab B. K., Van Embden J. D., Thole J. E., Kiessling R. The recombinant 65-kD heat shock protein of Mycobacterium bovis Bacillus Calmette-Guerin/M. tuberculosis is a target molecule for CD4+ cytotoxic T lymphocytes that lyse human monocytes. J Exp Med. 1988 Nov 1;168(5):1947–1952. doi: 10.1084/jem.168.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchenik A. E., Cole C., Russell B. W., Fischl M. A., Spira T. J., Snider D. E., Jr Tuberculosis, atypical mycobacteriosis, and the acquired immunodeficiency syndrome among Haitian and non-Haitian patients in south Florida. Ann Intern Med. 1984 Nov;101(5):641–645. doi: 10.7326/0003-4819-101-5-641. [DOI] [PubMed] [Google Scholar]
- Rickinson A. B., Rowe M., Hart I. J., Yao Q. Y., Henderson L. E., Rabin H., Epstein M. A. T-cell-mediated regression of "spontaneous" and of Epstein-Barr virus-induced B-cell transformation in vitro: studies with cyclosporin A. Cell Immunol. 1984 Sep;87(2):646–658. doi: 10.1016/0008-8749(84)90032-7. [DOI] [PubMed] [Google Scholar]
- Rook G. A. Progress in the immunology of the mycobacterioses. Clin Exp Immunol. 1987 Jul;69(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- Toman K. Bacterial persistence in leprosy. Int J Lepr Other Mycobact Dis. 1981 Jun;49(2):205–217. [PubMed] [Google Scholar]
- Wallace L. E., Moss D. J., Rickinson A. B., McMichael A. J., Epstein M. A. Cytotoxic T cell recognition of Epstein-Barr virus-infected B cells. II. Blocking studies with monoclonal antibodies to HLA determinants. Eur J Immunol. 1981 Sep;11(9):694–699. doi: 10.1002/eji.1830110905. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe S. N. Observations on the biochemical basis of ethanol metabolism by human macrophages. Alcohol Alcohol. 1986;21(1):57–63. [PubMed] [Google Scholar]
- Woan M. C., McGregor D. D., Forsum U. T cell-mediated cytotoxicity induced by Listeria monocytogenes. II. Specificity of cytolytic effector cells. J Immunol. 1981 Dec;127(6):2325–2330. [PubMed] [Google Scholar]


