Abstract
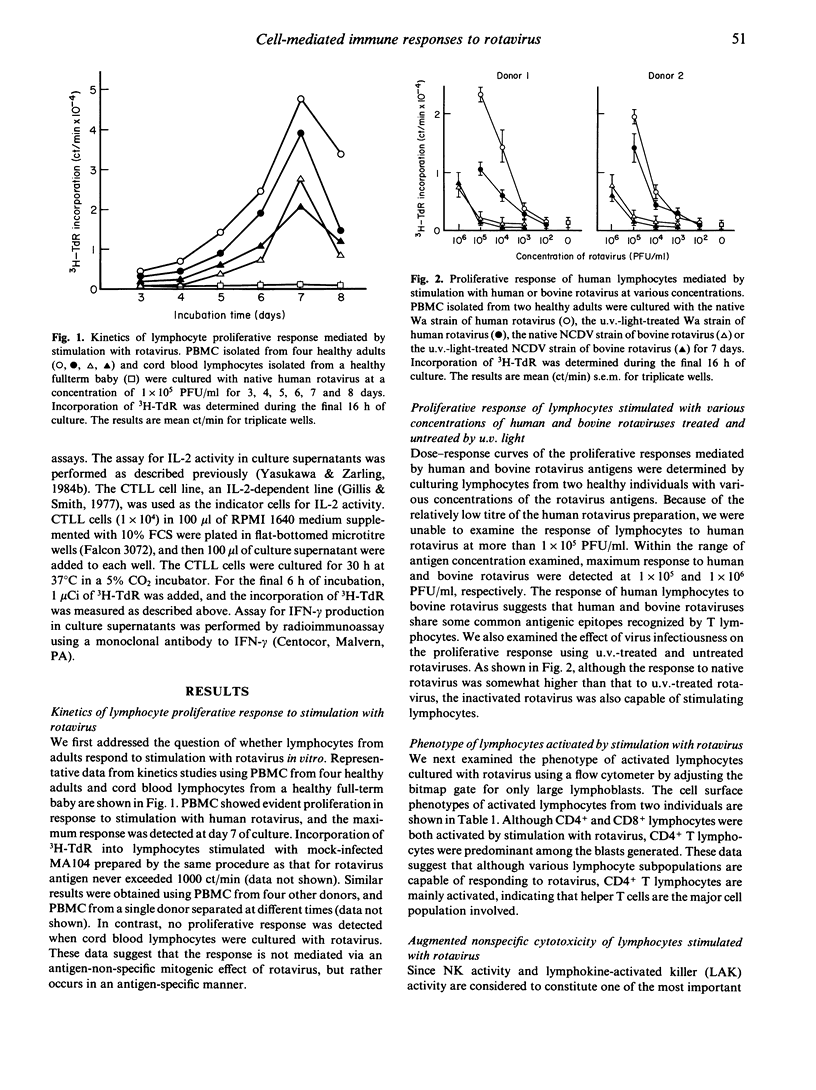
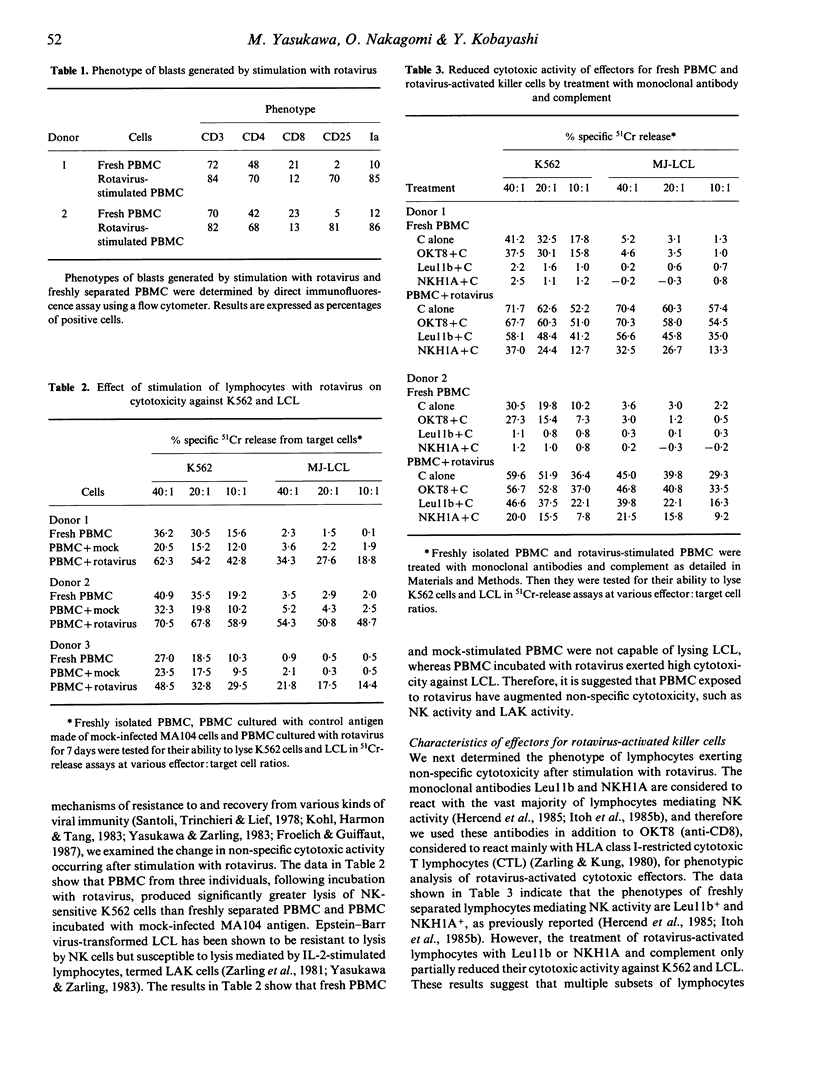
In vitro cell-mediated immune responses to rotavirus in humans were studied. Peripheral blood mononuclear cells (PBMC) of healthy adults proliferated in response to stimulation with the infectious and u.v.-inactivated Wa strain of human rotavirus, showing a maximum response on day 7 of culture; however, cord blood lymphocytes failed to respond to rotavirus. A cross-reactive proliferative response of PBMC detected by stimulation with the NCDV strain of bovine rotavirus suggests the existence of epitopes common to both human and bovine rotaviruses, which are recognized by human T lymphocytes. The phenotype of the majority of activated lymphocytes was CD3+4+8-, indicating that the cells mainly activated were helper T cells. Culture supernatants of PBMC stimulated with rotavirus contained interleukin-2 (IL-2) and interferon-gamma (IFN-gamma). In addition, PBMC stimulated with rotavirus demonstrated significantly enhanced cytotoxic activity against natural killer (NK) sensitive K562 cells as well as an NK-resistant Epstein-Barr virus-immortalized lymphoblastoid cell line (LCL). Treatment of PBMC with anti-CD16 or NKH1A monoclonal antibody, both of which react with most NK cells and lymphokine-activated killer cells and complement markedly reduced the cytotoxic activity against K562 and LCL. These results suggest that stimulation of human PBMC with rotavirus results in the production of lymphokines, such as IL-2 and IFN-gamma, by rotavirus-reactive helper T cells and that these lymphokines augment NK activity and generate other forms of non-specific cytotoxic human lymphocyte activity. These cell-mediated immune responses observed in the present in vitro study might play an important role in protection and recovery from rotavirus infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonneville M., Janeway C. A., Jr, Ito K., Haser W., Ishida I., Nakanishi N., Tonegawa S. Intestinal intraepithelial lymphocytes are a distinct set of gamma delta T cells. Nature. 1988 Dec 1;336(6198):479–481. doi: 10.1038/336479a0. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Borian F. E., Bell L. M., Modesto K., Gouvea V., Plotkin S. A. Protective effect of WC3 vaccine against rotavirus diarrhea in infants during a predominantly serotype 1 rotavirus season. J Infect Dis. 1988 Sep;158(3):570–587. doi: 10.1093/infdis/158.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst P. B., Clark D. A., Rosenthal K. L., Befus A. D., Bienenstock J. Detection and characterization of cytotoxic T lymphocyte precursors in the murine intestinal intraepithelial leukocyte population. J Immunol. 1986 Mar 15;136(6):2121–2126. [PubMed] [Google Scholar]
- Fitzgerald P. A., Mendelsohn M., Lopez C. Human natural killer cells limit replication of herpes simplex virus type 1 in vitro. J Immunol. 1985 Apr;134(4):2666–2672. [PubMed] [Google Scholar]
- Flores J., Nakagomi O., Nakagomi T., Glass R., Gorziglia M., Askaa J., Hoshino Y., Perez-Schael I., Kapikian A. Z. The role of rotaviruses in pediatric diarrhea. Pediatr Infect Dis. 1986 Jan-Feb;5(1 Suppl):S53–S62. doi: 10.1097/00006454-198601001-00011. [DOI] [PubMed] [Google Scholar]
- Flores J., Perez-Schael I., Gonzalez M., Garcia D., Perez M., Daoud N., Cunto W., Chanock R. M., Kapikian A. Z. Protection against severe rotavirus diarrhoea by rhesus rotavirus vaccine in Venezuelan infants. Lancet. 1987 Apr 18;1(8538):882–884. doi: 10.1016/s0140-6736(87)92858-3. [DOI] [PubMed] [Google Scholar]
- Froelich C. J., Guiffaut S. Lysis of human T cell leukemia virus infected T and B lymphoid cells by interleukin 2-activated killer cells. J Immunol. 1987 Dec 1;139(11):3637–3643. [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Goodman T., Lefrançois L. Expression of the gamma-delta T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988 Jun 30;333(6176):855–858. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- Grimm E. A., Mazumder A., Zhang H. Z., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982 Jun 1;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henney C. S., Kuribayashi K., Kern D. E., Gillis S. Interleukin-2 augments natural killer cell activity. Nature. 1981 May 28;291(5813):335–338. doi: 10.1038/291335a0. [DOI] [PubMed] [Google Scholar]
- Hercend T., Griffin J. D., Bensussan A., Schmidt R. E., Edson M. A., Brennan A., Murray C., Daley J. F., Schlossman S. F., Ritz J. Generation of monoclonal antibodies to a human natural killer clone. Characterization of two natural killer-associated antigens, NKH1A and NKH2, expressed on subsets of large granular lymphocytes. J Clin Invest. 1985 Mar;75(3):932–943. doi: 10.1172/JCI111794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Itoh K., Shiiba K., Shimizu Y., Suzuki R., Kumagai K. Generation of activated killer (AK) cells by recombinant interleukin 2 (rIL 2) in collaboration with interferon-gamma (IFN-gamma). J Immunol. 1985 May;134(5):3124–3129. [PubMed] [Google Scholar]
- Itoh K., Tilden A. B., Kumagai K., Balch C. M. Leu-11+ lymphocytes with natural killer (NK) activity are precursors of recombinant interleukin 2 (rIL 2)-induced activated killer (AK) cells. J Immunol. 1985 Feb;134(2):802–807. [PubMed] [Google Scholar]
- Klein J. R., Kagnoff M. F. Nonspecific recruitment of cytotoxic effector cells in the intestinal mucosa of antigen-primed mice. J Exp Med. 1984 Dec 1;160(6):1931–1936. doi: 10.1084/jem.160.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl S., Harmon M. W., Tang J. P. Cytokine-stimulated human natural killer cytotoxicity: response to rotavirus-infected cells. Pediatr Res. 1983 Nov;17(11):868–872. doi: 10.1203/00006450-198311000-00006. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Morrison L. A., Braciale V. L., Braciale T. J. Antigen form influences induction and frequency of influenza-specific class I and class II MHC-restricted cytolytic T lymphocytes. J Immunol. 1988 Jul 15;141(2):363–368. [PubMed] [Google Scholar]
- Nakagomi O., Nakagomi T., Hoshino Y., Flores J., Kapikian A. Z. Genetic analysis of a human rotavirus that belongs to subgroup I but has an RNA pattern typical of subgroup II human rotaviruses. J Clin Microbiol. 1987 Jul;25(7):1159–1164. doi: 10.1128/jcm.25.7.1159-1164.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F. Protection against rotavirus-induced gastroenteritis in a murine model by passively acquired gastrointestinal but not circulating antibodies. J Virol. 1985 Apr;54(1):58–64. doi: 10.1128/jvi.54.1.58-64.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Dudzik K. I. Rotavirus-specific cytotoxic T lymphocytes cross-react with target cells infected with different rotavirus serotypes. J Virol. 1988 Jan;62(1):127–131. doi: 10.1128/jvi.62.1.127-131.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortaldo J. R., Mason A., Overton R. Lymphokine-activated killer cells. Analysis of progenitors and effectors. J Exp Med. 1986 Oct 1;164(4):1193–1205. doi: 10.1084/jem.164.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riepenhoff-Talty M., Bogger-Goren S., Li P., Carmody P. J., Barrett H. J., Ogra P. L. Development of serum and intestinal antibody response to rotavirus after naturally acquired rotavirus infection in man. J Med Virol. 1981;8(3):215–222. doi: 10.1002/jmv.1890080309. [DOI] [PubMed] [Google Scholar]
- Sack D. A., Gilman R. H., Kapikian A. Z., Aziz K. M. Seroepidemiology of rotavirus infection in rural Bangladesh. J Clin Microbiol. 1980 May;11(5):530–532. doi: 10.1128/jcm.11.5.530-532.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoli D., Trinchieri G., Lief F. S. Cell-mediated cytotoxicity against virus-infected target cells in humans. I. Characterization of the effector lymphocyte. J Immunol. 1978 Aug;121(2):526–531. [PubMed] [Google Scholar]
- Schoub B. D., Prozesky O. W., Lecatsas G., Oosthuizen R. The role of breast-feeding in the prevention of rotavirus infection. J Med Microbiol. 1978 Feb;11(1):25–31. doi: 10.1099/00222615-11-1-25. [DOI] [PubMed] [Google Scholar]
- Sugden B., Mark W. Clonal transformation of adult human leukocytes by Epstein-Barr virus. J Virol. 1977 Sep;23(3):503–508. doi: 10.1128/jvi.23.3.503-508.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totterdell B. M., Banatvala J. E., Chrystie I. L., Ball G., Cubitt W. D. Systemic lymphoproliferative responses to rotavirus. J Med Virol. 1988 May;25(1):37–44. doi: 10.1002/jmv.1890250106. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., D'Hondt E., Delem A., André F. E., Zissis G. Protection of infants against rotavirus diarrhoea by RIT 4237 attenuated bovine rotavirus strain vaccine. Lancet. 1984 May 5;1(8384):977–981. doi: 10.1016/s0140-6736(84)92323-7. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., Delem A., d'Hondt E., André F. E., Beards G. M., Flewett T. H. Clinical efficacy of the RIT 4237 live attenuated bovine rotavirus vaccine in infants vaccinated before a rotavirus epidemic. J Pediatr. 1985 Aug;107(2):189–194. doi: 10.1016/s0022-3476(85)80123-2. [DOI] [PubMed] [Google Scholar]
- Yasukawa M., Kobayashi Y. Inhibition of herpes simplex virus replication in vitro by human cytotoxic T cell clones and natural killer cell clones. J Gen Virol. 1985 Oct;66(Pt 10):2225–2229. doi: 10.1099/0022-1317-66-10-2225. [DOI] [PubMed] [Google Scholar]
- Yasukawa M., Zarling J. M. Autologous herpes simplex virus-infected cells are lysed by human natural killer cells. J Immunol. 1983 Oct;131(4):2011–2016. [PubMed] [Google Scholar]
- Zarling J. M., Dierckins M. S., Sevenich E. A., Clouse K. A. Stimulation with autologous lymphoblastoid cell lines: lysis of Epstein-Barr virus-positive and -negative cell lines by two phenotypically distinguishable effector cell populations. J Immunol. 1981 Nov;127(5):2118–2123. [PubMed] [Google Scholar]
- Zarling J. M., Kung P. C. Monoclonal antibodies which distinguish between human NK cells and cytotoxic T lymphocytes. Nature. 1980 Nov 27;288(5789):394–396. doi: 10.1038/288394a0. [DOI] [PubMed] [Google Scholar]


