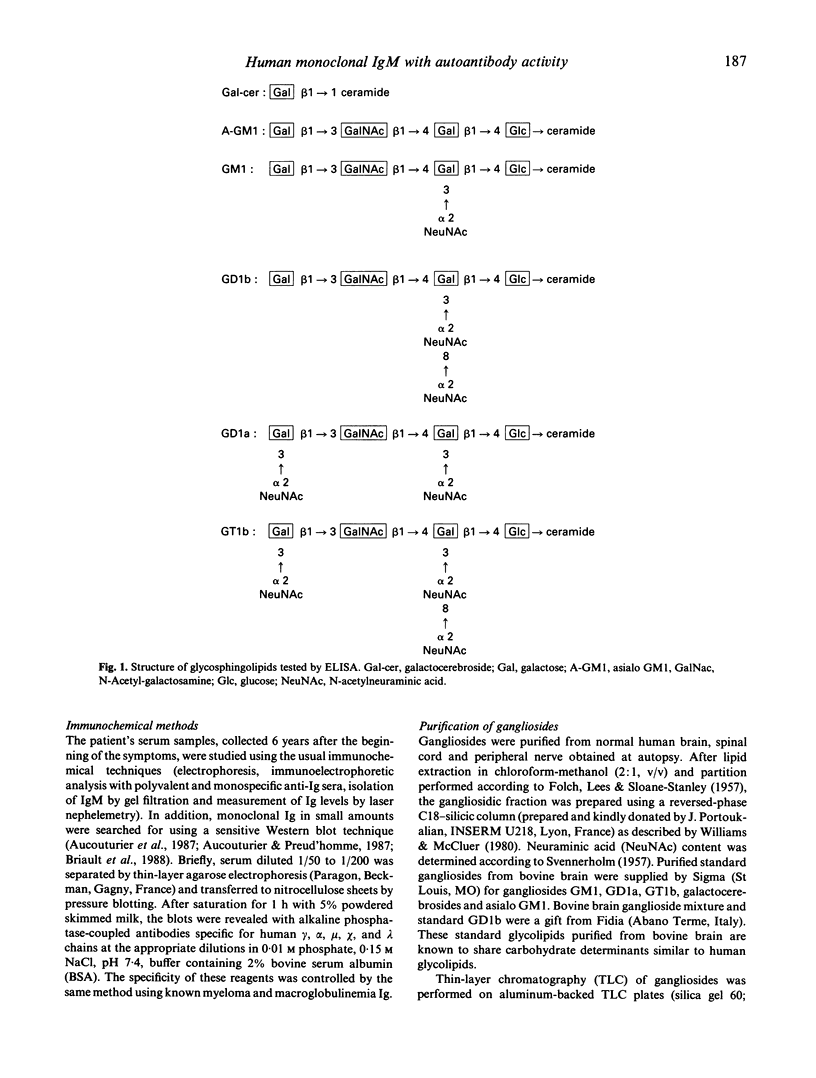
Abstract
Small amounts of oligoclonal immunoglobulins were detected by Western blotting in the serum from a patient with motor neuron syndrome. The prominent one, a monoclonal IgM lambda, reacted strongly with the gangliosides GM1 and GD1b and more weakly with asialo GM1, as shown by immunoenzymatic staining of thin-layer chromatograms of gangliosides, ELISA on purified glycolipid coats and immunoadsorption with purified GM1. Affinity-chromatography with purified GM1 resulted in the purification of monoclonal IgM lambda. This purified IgM and its Fab fragments showed the same pattern of reactivity with gangliosides as that observed with whole serum. Such monoclonal IgM could be responsible for motor neuron diseases in some patients with overt or barely detectable monoclonal gammopathies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun P. E., Frail D. E., Latov N. Myelin-associated glycoprotein is the antigen for a monoclonal IgM in polyneuropathy. J Neurochem. 1982 Nov;39(5):1261–1265. doi: 10.1111/j.1471-4159.1982.tb12563.x. [DOI] [PubMed] [Google Scholar]
- Briault S., Courtois-Capella M., Duarte F., Aucouturier P., Preud'Homme J. L. Isotypy of serum monoclonal immunoglobulins in human immunodeficiency virus-infected adults. Clin Exp Immunol. 1988 Nov;74(2):182–184. [PMC free article] [PubMed] [Google Scholar]
- Chou D. K., Ilyas A. A., Evans J. E., Costello C., Quarles R. H., Jungalwala F. B. Structure of sulfated glucuronyl glycolipids in the nervous system reacting with HNK-1 antibody and some IgM paraproteins in neuropathy. J Biol Chem. 1986 Sep 5;261(25):11717–11725. [PubMed] [Google Scholar]
- Chou K. H., Ilyas A. A., Evans J. E., Quarles R. H., Jungalwala F. B. Structure of a glycolipid reacting with monoclonal IgM in neuropathy and with HNK-1. Biochem Biophys Res Commun. 1985 Apr 16;128(1):383–388. doi: 10.1016/0006-291x(85)91690-0. [DOI] [PubMed] [Google Scholar]
- Coulon-Morelec M. J. Purification des anticorps anti-haptène lipidique. Ann Inst Pasteur (Paris) 1972 Nov;123(5):619–640. [PubMed] [Google Scholar]
- Dellagi K., Brouet J. C., Perreau J., Paulin D. Human monoclonal IgM with autoantibody activity against intermediate filaments. Proc Natl Acad Sci U S A. 1982 Jan;79(2):446–450. doi: 10.1073/pnas.79.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T., Scott D. D., Stewart S. S., Kundu S. K., Marcus D. M. Antibodies to glycosphingolipids in patients with multiple sclerosis and SLE. J Immunol. 1984 Apr;132(4):1793–1797. [PubMed] [Google Scholar]
- Engelhardt J., Joo F. An immune-mediated guinea pig model for lower motor neuron disease. J Neuroimmunol. 1986 Oct;12(4):279–290. doi: 10.1016/0165-5728(86)90034-2. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Freddo L., Yu R. K., Latov N., Donofrio P. D., Hays A. P., Greenberg H. S., Albers J. W., Allessi A. G., Keren D. Gangliosides GM1 and GD1b are antigens for IgM M-protein in a patient with motor neuron disease. Neurology. 1986 Apr;36(4):454–458. doi: 10.1212/wnl.36.4.454. [DOI] [PubMed] [Google Scholar]
- Haas D. C., Tatum A. H. Plasmapheresis alleviates neuropathy accompanying IgM anti-myelin-associated glycoprotein paraproteinemia. Ann Neurol. 1988 Apr;23(4):394–396. doi: 10.1002/ana.410230415. [DOI] [PubMed] [Google Scholar]
- Harpin M. L., Coulon-Morelec M. J., Yeni P., Danon F., Baumann N. Direct sensitive immunocharacterization of gangliosides on plastic thin-layer plates using peroxidase staining. J Immunol Methods. 1985 Apr 8;78(1):135–141. doi: 10.1016/0022-1759(85)90336-9. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y., Suzuki T., Suzuki Y., Taki T., Matsumoto M., Higashi H., Kato S. A new method for purification of anti-glycosphingolipid antibody. Avian anti-hematoside (NeuGc) antibody. J Biochem. 1983 Jul;94(1):327–330. doi: 10.1093/oxfordjournals.jbchem.a134350. [DOI] [PubMed] [Google Scholar]
- Ilyas A. A., Quarles R. H., Dalakas M. C., Brady R. O. Polyneuropathy with monoclonal gammopathy: glycolipids are frequently antigens for IgM paraproteins. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6697–6700. doi: 10.1073/pnas.82.19.6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyas A. A., Quarles R. H., Dalakas M. C., Fishman P. H., Brady R. O. Monoclonal IgM in a patient with paraproteinemic polyneuropathy binds to gangliosides containing disialosyl groups. Ann Neurol. 1985 Dec;18(6):655–659. doi: 10.1002/ana.410180605. [DOI] [PubMed] [Google Scholar]
- Ilyas A. A., Quarles R. H., MacIntosh T. D., Dobersen M. J., Trapp B. D., Dalakas M. C., Brady R. O. IgM in a human neuropathy related to paraproteinemia binds to a carbohydrate determinant in the myelin-associated glycoprotein and to a ganglioside. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1225–1229. doi: 10.1073/pnas.81.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Latov N. Monoclonal IgM in two patients with motor neuron disease bind to the carbohydrate antigens Gal(beta 1-3)GalNAc and Gal(beta 1-3)GlcNAc. J Neuroimmunol. 1988 Sep;19(3):245–253. doi: 10.1016/0165-5728(88)90006-9. [DOI] [PubMed] [Google Scholar]
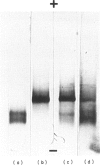
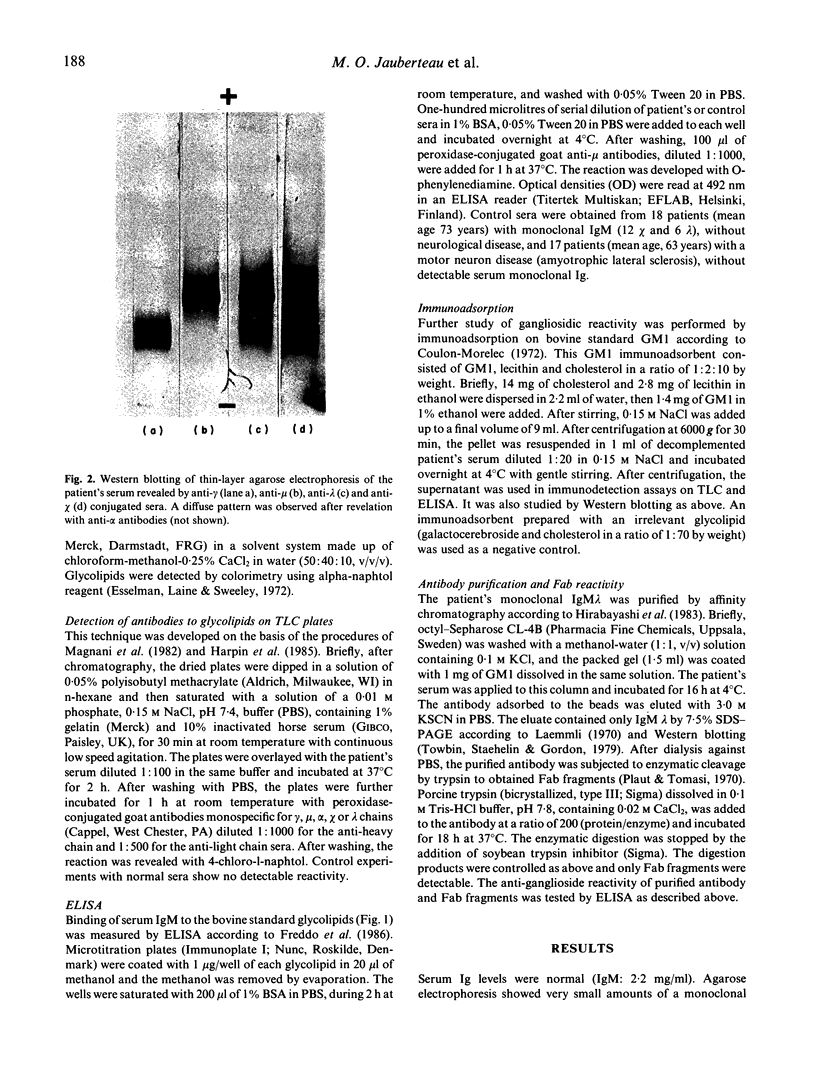
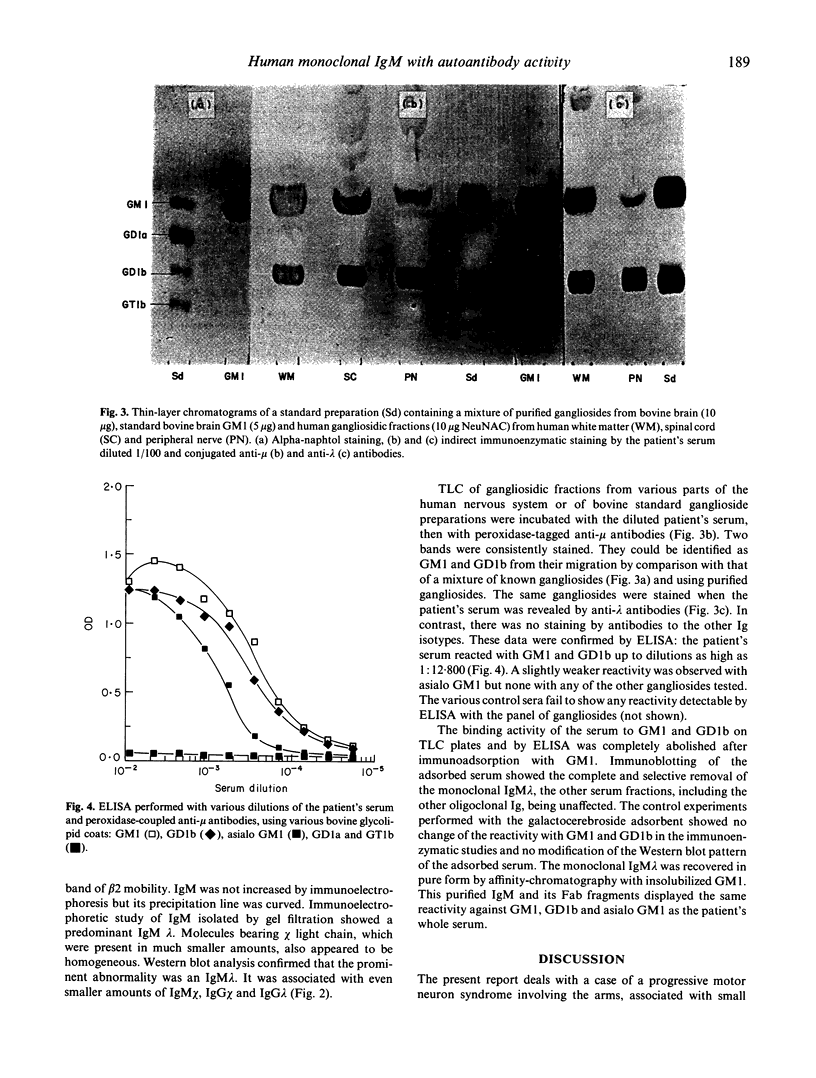
- Jauberteau M. O., Henin D., Bouche P., Vallat J. M., Dumas M., Dellagi S., Leger J. M., Harpin M. L., Ratinahirana H., Chaunu M. P. Etude des anticorps antiglycolipides au cours des dysglobulinémies monoclonales à IgM associées à une neuropathie périphérique. Rev Neurol (Paris) 1988;144(8-9):474–480. [PubMed] [Google Scholar]
- Jauberteau M. O., Younes-Chennoufi B., Rigaud M., Baumann N. IgM gammopathy and polyneuropathy react with an antigenic glycolipid present in human central nervous system. Neurosci Lett. 1989 Feb 13;97(1-2):181–184. doi: 10.1016/0304-3940(89)90160-2. [DOI] [PubMed] [Google Scholar]
- Kusunoki S., Shimizu T., Matsumura K., Maemura K., Mannen T. Motor dominant neuropathy and IgM paraproteinemia: the IgM M-protein binds to specific gangliosides. J Neuroimmunol. 1989 Feb;21(2-3):177–181. doi: 10.1016/0165-5728(89)90173-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Latov N., Hays A. P., Donofrio P. D., Liao J., Ito H., McGinnis S., Konstadoulakis M., Freddo L., Shy M. E., Manoussos K. Monoclonal IgM with unique specificity to gangliosides GM1 and GD1b and to lacto-N-tetraose associated with human motor neuron disease. Neurology. 1988 May;38(5):763–768. doi: 10.1212/wnl.38.5.763. [DOI] [PubMed] [Google Scholar]
- Magnani J. L., Nilsson B., Brockhaus M., Zopf D., Steplewski Z., Koprowski H., Ginsburg V. A monoclonal antibody-defined antigen associated with gastrointestinal cancer is a ganglioside containing sialylated lacto-N-fucopentaose II. J Biol Chem. 1982 Dec 10;257(23):14365–14369. [PubMed] [Google Scholar]
- Miyatani N., Baba H., Sato S., Nakamura K., Yuasa T., Miyatake T. Antibody to sialosyllactosaminylparagloboside in a patient with IgM paraproteinemia and polyradiculoneuropathy. J Neuroimmunol. 1987 Mar;14(2):189–196. doi: 10.1016/0165-5728(87)90053-1. [DOI] [PubMed] [Google Scholar]
- Nardelli E., Steck A. J., Barkas T., Schluep M., Jerusalem F. Motor neuron syndrome and monoclonal IgM with antibody activity against gangliosides GM1 and GD1b. Ann Neurol. 1988 May;23(5):524–528. doi: 10.1002/ana.410230517. [DOI] [PubMed] [Google Scholar]
- Pestronk A., Adams R. N., Clawson L., Cornblath D., Kuncl R. W., Griffin D., Drachman D. B. Serum antibodies to GM1 ganglioside in amyotrophic lateral sclerosis. Neurology. 1988 Sep;38(9):1457–1461. doi: 10.1212/wnl.38.9.1457. [DOI] [PubMed] [Google Scholar]
- Pestronk A., Cornblath D. R., Ilyas A. A., Baba H., Quarles R. H., Griffin J. W., Alderson K., Adams R. N. A treatable multifocal motor neuropathy with antibodies to GM1 ganglioside. Ann Neurol. 1988 Jul;24(1):73–78. doi: 10.1002/ana.410240113. [DOI] [PubMed] [Google Scholar]
- Plaut A. G., Tomasi T. B., Jr Immunoglobulin M: pentameric Fcmu fragments released by trypsin at higher temperatures. Proc Natl Acad Sci U S A. 1970 Feb;65(2):318–322. doi: 10.1073/pnas.65.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarles R. H. Myelin-associated glycoprotein in development and disease. Dev Neurosci. 1983;6(6):285–303. doi: 10.1159/000112356. [DOI] [PubMed] [Google Scholar]
- Ritchie T. C., Fabian R. H., Choate J. V., Coulter J. D. Axonal transport of monoclonal antibodies. J Neurosci. 1986 Apr;6(4):1177–1184. doi: 10.1523/JNEUROSCI.06-04-01177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. A., McCluer R. H. The use of Sep-Pak C18 cartridges during the isolation of gangliosides. J Neurochem. 1980 Jul;35(1):266–269. doi: 10.1111/j.1471-4159.1980.tb12515.x. [DOI] [PubMed] [Google Scholar]