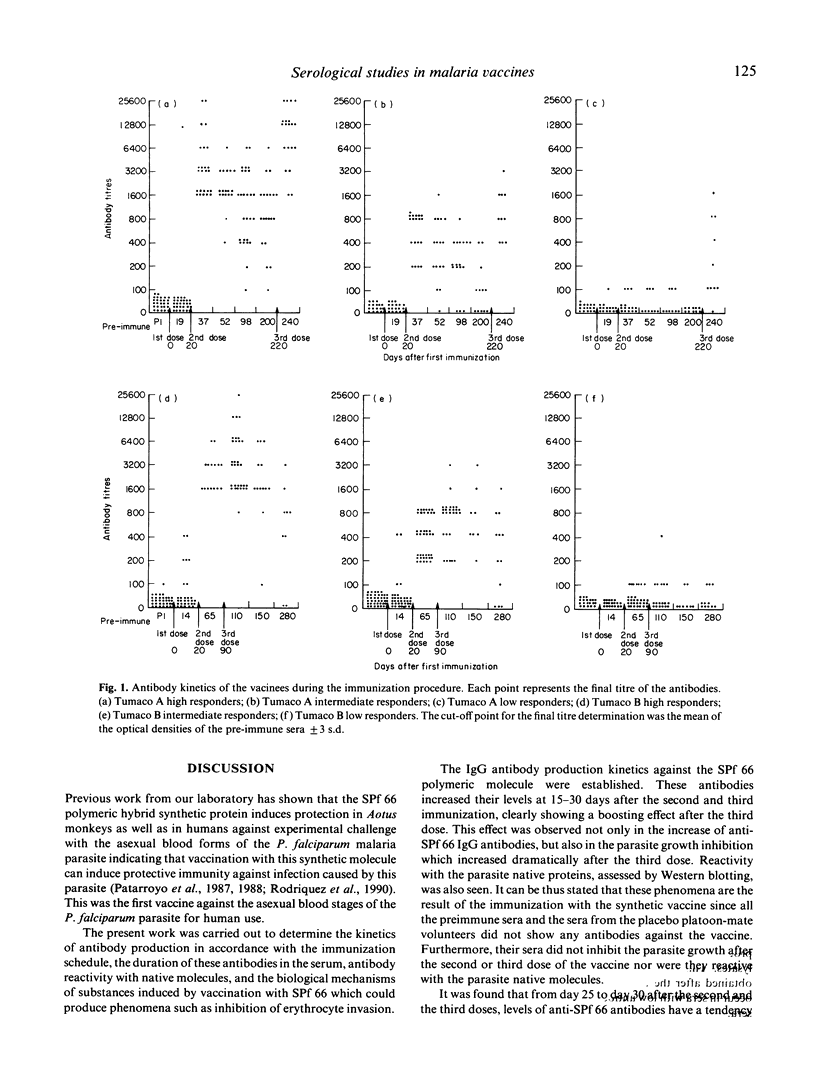
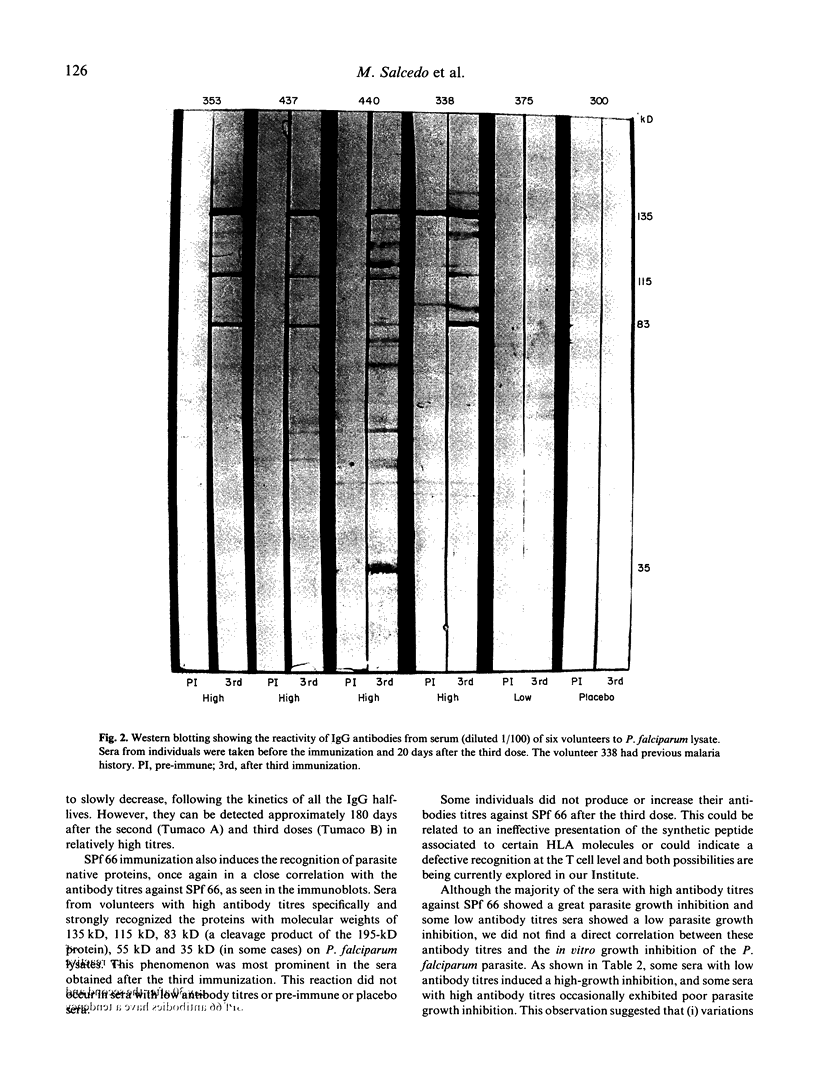
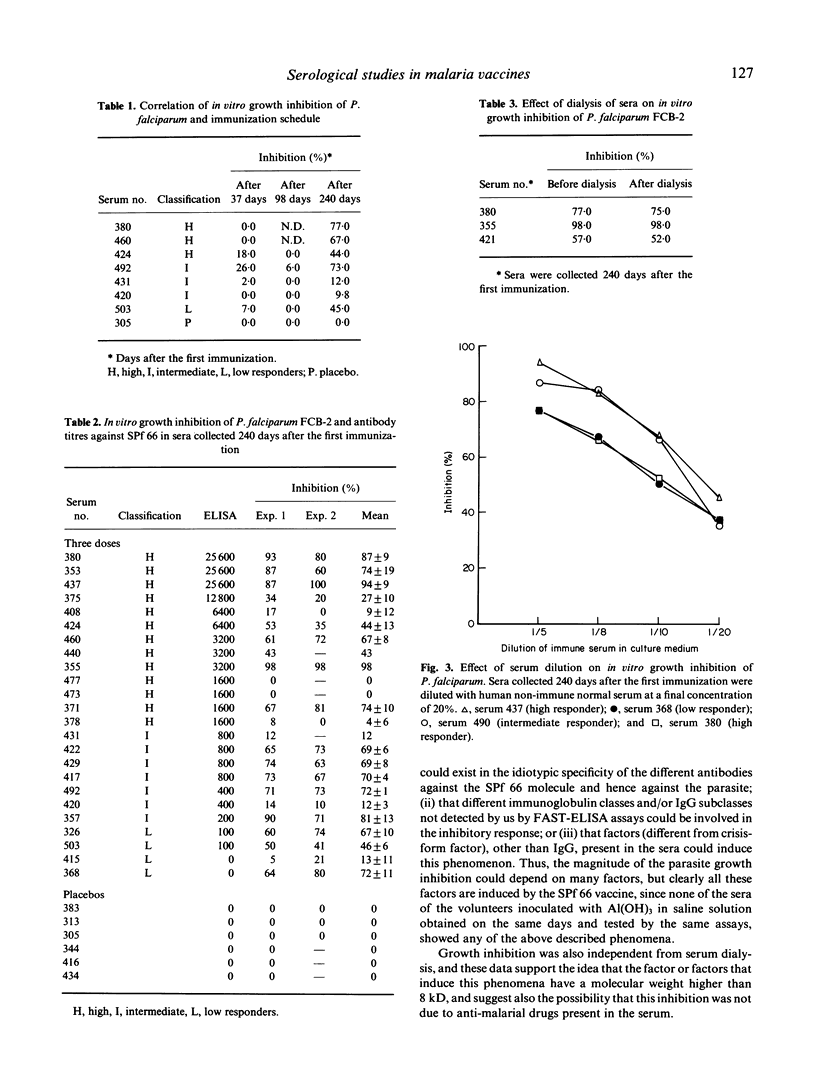
Abstract
A synthetic vaccine against the asexual blood stages of P. falciparum, the SPf 66 synthetic hybrid polymer, composed of peptides derived from three merozoite membrane proteins as well as one peptide from the sporozoite CS protein, has been developed by our group and tested in different protection assays in Aotus monkeys as well as in human volunteers. This study evaluates the humoral immune response induced by the SPf 66 protein vaccination in adult human volunteers from the Colombian Pacific coast as follows: determination of specific IgG antibody levels against SPf 66 by FAST-ELISA after each immunization; analysis of antibody reactivity with P. falciparum schizont lysates by immunoblots; and determination of the in vitro parasite growth inhibition. A clear boosting effect, dependent on time and dose, was observed in the antibody production kinetics. These antibodies also specifically recognize three proteins of the P. falciparum schizont lysate corresponding to the molecular weights of the proteins from which the amino acid sequence was derived. These sera were also capable of markedly inhibiting in vitro parasite growth.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou W. R., Hoffman S. L., Sherwood J. A., Hollingdale M. R., Neva F. A., Hockmeyer W. T., Gordon D. M., Schneider I., Wirtz R. A., Young J. F. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet. 1987 Jun 6;1(8545):1277–1281. doi: 10.1016/s0140-6736(87)90540-x. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Brown G. V., Anders R. F., Mitchell G. F., Heywood P. F. Target antigens of purified human immunoglobulins which inhibit growth of Plasmodium falciparum in vitro. Nature. 1982 Jun 17;297(5867):591–593. doi: 10.1038/297591a0. [DOI] [PubMed] [Google Scholar]
- Campbell G. H., Aley S. B., Ballou W. R., Hall T., Hockmeyer W. T., Hoffman S. L., Hollingdale M. R., Howard R. J., Lyon J. A., Nardin E. H. Use of synthetic and recombinant peptides in the study of host-parasite interactions in the malarias. Am J Trop Med Hyg. 1987 Nov;37(3):428–444. doi: 10.4269/ajtmh.1987.37.428. [DOI] [PubMed] [Google Scholar]
- Collins W. E., Pappaioanou M., Anders R. F., Campbell G. H., Brown G. V., Kemp D. J., Broderson J. R., Coppel R. L., Skinner J. C., Procell P. M. Immunization trials with the ring-infected erythrocyte surface antigen of Plasmodium falciparum in owl monkeys (Aotus vociferans). Am J Trop Med Hyg. 1988 Mar;38(2):268–282. doi: 10.4269/ajtmh.1988.38.268. [DOI] [PubMed] [Google Scholar]
- Grumet F. C. Genetic control of the immune response. A selective defect in immunologic (IgG) memory in nonresponder mice. J Exp Med. 1972 Jan;135(1):110–125. doi: 10.1084/jem.135.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet F. C., Mitchell G. F., McDevitt H. O. Genetic control of specific immune responses in inbred mice. Ann N Y Acad Sci. 1971 Dec 31;190:170–177. doi: 10.1111/j.1749-6632.1971.tb13532.x. [DOI] [PubMed] [Google Scholar]
- Herrington D. A., Clyde D. F., Losonsky G., Cortesia M., Murphy J. R., Davis J., Baqar S., Felix A. M., Heimer E. P., Gillessen D. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature. 1987 Jul 16;328(6127):257–259. doi: 10.1038/328257a0. [DOI] [PubMed] [Google Scholar]
- Houghten R. A. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui G. S., Siddiqui W. A. Serum from Pf195 protected Aotus monkeys inhibit Plasmodium falciparum growth in vitro. Exp Parasitol. 1987 Dec;64(3):519–522. doi: 10.1016/0014-4894(87)90068-3. [DOI] [PubMed] [Google Scholar]
- Jepsen S. Inhibition of in vitro growth of Plasmodium falciparum by purified antimalarial human IgG antibodies. Isolation of target antigens from culture supernatants. Scand J Immunol. 1983 Dec;18(6):567–571. doi: 10.1111/j.1365-3083.1983.tb00893.x. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Mitchell G. H., Butcher G. A., Voller A., Cohen S. The effect of human immune IgG on the in vitro development of Plasmodium falciparum. Parasitology. 1976 Apr;72(2):149–162. doi: 10.1017/s0031182000048459. [DOI] [PubMed] [Google Scholar]
- Nussenzweig R. S., Nussenzweig V. Development of sporozoite vaccines. Philos Trans R Soc Lond B Biol Sci. 1984 Nov 13;307(1131):117–128. doi: 10.1098/rstb.1984.0113. [DOI] [PubMed] [Google Scholar]
- Patarroyo M. E., Romero P., Torres M. L., Clavijo P., Moreno A., Martínez A., Rodríguez R., Guzman F., Cabezas E. Induction of protective immunity against experimental infection with malaria using synthetic peptides. Nature. 1987 Aug 13;328(6131):629–632. doi: 10.1038/328629a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez R., Moreno A., Guzman F., Calvo M., Patarroyo M. E. Studies in owl monkeys leading to the development of a synthetic vaccine against the asexual blood stages of Plasmodium falciparum. Am J Trop Med Hyg. 1990 Oct;43(4):339–354. doi: 10.4269/ajtmh.1990.43.339. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Wåhlin B., Wahlgren M., Perlmann H., Berzins K., Björkman A., Patarroyo M. E., Perlmann P. Human antibodies to a Mr 155,000 Plasmodium falciparum antigen efficiently inhibit merozoite invasion. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7912–7916. doi: 10.1073/pnas.81.24.7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolg J. W., MacLeod A. J., Dickson I. H., Scaife J. G. Plasmodium falciparum: modifications of the in vitro culture conditions improving parasitic yields. J Parasitol. 1982 Dec;68(6):1072–1080. [PubMed] [Google Scholar]


