Abstract
Protein-containing surfactants of human and animal origin are being used increasingly to treat neonatal and adult respiratory distress syndromes. This trend led us to examine the antigenicity of two important preparations of animal surfactant, cow lung surfactant extract (CLSE) and a porcine surfactant preparation, Curosurf. We describe here 15 monoclonal antibodies against Curosurf and four against CLSE. Antibodies were studied by Western blot analysis to determine their ability to recognize protein components of their respective surfactant preparations. They were also tested for their ability to inactivate surfactant in vitro, assayed using the pulsating bubble surfactometer. Several antibodies directed against CLSE or Curosurf functionally inactivate the surfactant to which they were raised. We determined the degree of immunologic cross-reactivity between antibodies directed to CLSE and Curosurf against the other surfactant and also against human surfactant, both by Western blot and by examining functional inactivation in vitro. Antibodies to these animal surfactants that are commonly used therapeutically may inactivate the specific animal surfactant to which they were raised, as well as human and other surfactants. Generally, when antibodies inactivate surfactant from more than one animal species, they inactivate heterologous surfactants comparably to the extent to which they inactivate the surfactant to which they are directed. Immune complexes between anti-surfactant antibodies and surfactant have been described in the course of neonatal respiratory distress syndrome. The potential pathophysiological importance of anti-surfactant antibodies may therefore lie in their ability to inactivate administered surfactant, other similar surfactants and endogenous surfactant. In so doing, these antibodies may potentiate surfactant deficiency or pulmonary injury initiated by other stimuli.
Full text
PDF

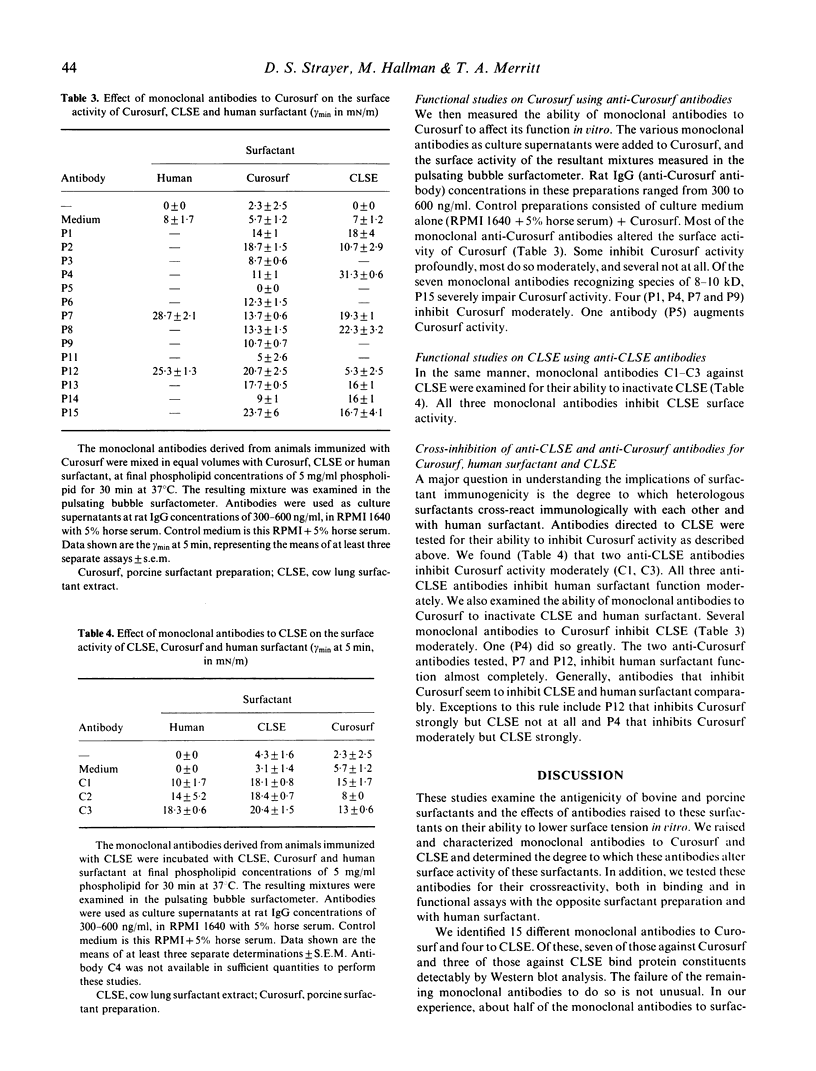


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVERY M. E., MEAD J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child. 1959 May;97(5 Pt 1):517–523. doi: 10.1001/archpedi.1959.02070010519001. [DOI] [PubMed] [Google Scholar]
- Curstedt T., Jörnvall H., Robertson B., Bergman T., Berggren P. Two hydrophobic low-molecular-mass protein fractions of pulmonary surfactant. Characterization and biophysical activity. Eur J Biochem. 1987 Oct 15;168(2):255–262. doi: 10.1111/j.1432-1033.1987.tb13414.x. [DOI] [PubMed] [Google Scholar]
- Enhorning G. Pulsating bubble technique for evaluating pulmonary surfactant. J Appl Physiol Respir Environ Exerc Physiol. 1977 Aug;43(2):198–203. doi: 10.1152/jappl.1977.43.2.198. [DOI] [PubMed] [Google Scholar]
- Hallman M., Merritt T. A., Schneider H., Epstein B. L., Mannino F., Edwards D. K., Gluck L. Isolation of human surfactant from amniotic fluid and a pilot study of its efficacy in respiratory distress syndrome. Pediatrics. 1983 Apr;71(4):473–482. [PubMed] [Google Scholar]
- Hawgood S., Benson B. J., Hamilton R. L., Jr Effects of a surfactant-associated protein and calcium ions on the structure and surface activity of lung surfactant lipids. Biochemistry. 1985 Jan 1;24(1):184–190. doi: 10.1021/bi00322a026. [DOI] [PubMed] [Google Scholar]
- Horbar J. D., Soll R. F., Sutherland J. M., Kotagal U., Philip A. G., Kessler D. L., Little G. A., Edwards W. H., Vidyasagar D., Raju T. N. A multicenter randomized, placebo-controlled trial of surfactant therapy for respiratory distress syndrome. N Engl J Med. 1989 Apr 13;320(15):959–965. doi: 10.1056/NEJM198904133201502. [DOI] [PubMed] [Google Scholar]
- Kendig J. W., Sinkin R. A. The effect of surfactant replacement therapy on conditions associated with respiratory distress syndrome: patent ductus arteriosus, intraventricular hemorrhage, and bronchopulmonary dysplasia. Semin Perinatol. 1988 Jul;12(3):255–258. [PubMed] [Google Scholar]
- Merritt T. A., Hallman M., Bloom B. T., Berry C., Benirschke K., Sahn D., Key T., Edwards D., Jarvenpaa A. L., Pohjavuori M. Prophylactic treatment of very premature infants with human surfactant. N Engl J Med. 1986 Sep 25;315(13):785–790. doi: 10.1056/NEJM198609253151301. [DOI] [PubMed] [Google Scholar]
- Merritt T. A., Hallman M. Surfactant replacement. A new era with many challenges for neonatal medicine. Am J Dis Child. 1988 Dec;142(12):1333–1339. doi: 10.1001/archpedi.1988.02150120087047. [DOI] [PubMed] [Google Scholar]
- Morley C. J. Surfactant substitution in the newborn by application of artificial surfactant. J Perinat Med. 1987;15(5):469–478. doi: 10.1515/jpme.1987.15.5.469. [DOI] [PubMed] [Google Scholar]
- Possmayer F. A proposed nomenclature for pulmonary surfactant-associated proteins. Am Rev Respir Dis. 1988 Oct;138(4):990–998. doi: 10.1164/ajrccm/138.4.990. [DOI] [PubMed] [Google Scholar]
- Revak S. D., Merritt T. A., Degryse E., Stefani L., Courtney M., Hallman M., Cochrane C. G. Use of human surfactant low molecular weight apoproteins in the reconstitution of surfactant biologic activity. J Clin Invest. 1988 Mar;81(3):826–833. doi: 10.1172/JCI113391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revak S. D., Merritt T. A., Hallman M., Cochrane C. G. Reconstitution of surfactant activity using purified human apoprotein and phospholipids measured in vitro and in vivo. Am Rev Respir Dis. 1986 Dec;134(6):1258–1265. doi: 10.1164/arrd.1986.134.5.1258. [DOI] [PubMed] [Google Scholar]
- Strayer D. S., Hallman M., Merritt T. A. Immunogenicity of surfactant. I. Human alveolar surfactant. Clin Exp Immunol. 1991 Jan;83(1):35–40. doi: 10.1111/j.1365-2249.1991.tb05584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayer D. S., Merritt T. A., Lwebuga-Mukasa J., Hallman M. Surfactant-anti-surfactant immune complexes in infants with respiratory distress syndrome. Am J Pathol. 1986 Feb;122(2):353–362. [PMC free article] [PubMed] [Google Scholar]
- Strayer D. S., Merritt T. A., Makunike C., Hallman M. Antigenicity of low molecular weight surfactant species. Am J Pathol. 1989 Apr;134(4):723–732. [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Curstedt T., Grossmann G., Kobayashi T., Nilsson R., Nohara K., Robertson B. The role of the low-molecular weight (less than or equal to 15,000 daltons) apoproteins of pulmonary surfactant. Eur J Respir Dis. 1986 Nov;69(5):336–345. [PubMed] [Google Scholar]
- Warr R. G., Hawgood S., Buckley D. I., Crisp T. M., Schilling J., Benson B. J., Ballard P. L., Clements J. A., White R. T. Low molecular weight human pulmonary surfactant protein (SP5): isolation, characterization, and cDNA and amino acid sequences. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7915–7919. doi: 10.1073/pnas.84.22.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsett J. A., Hull W. M., Ohning B., Ross G., Weaver T. E. Immunologic identification of a pulmonary surfactant-associated protein of molecular weight = 6000 daltons. Pediatr Res. 1986 Aug;20(8):744–749. doi: 10.1203/00006450-198608000-00009. [DOI] [PubMed] [Google Scholar]
- Whitsett J. A., Ohning B. L., Ross G., Meuth J., Weaver T., Holm B. A., Shapiro D. L., Notter R. H. Hydrophobic surfactant-associated protein in whole lung surfactant and its importance for biophysical activity in lung surfactant extracts used for replacement therapy. Pediatr Res. 1986 May;20(5):460–467. doi: 10.1203/00006450-198605000-00016. [DOI] [PubMed] [Google Scholar]
- Yu S. H., Possmayer F. Reconstitution of surfactant activity by using the 6 kDa apoprotein associated with pulmonary surfactant. Biochem J. 1986 May 15;236(1):85–89. doi: 10.1042/bj2360085. [DOI] [PMC free article] [PubMed] [Google Scholar]




