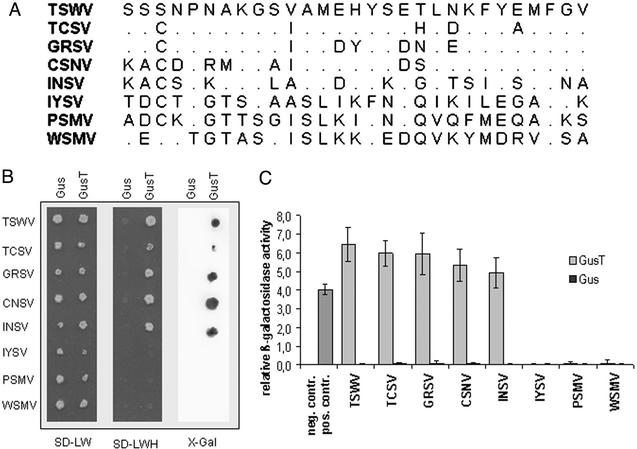
Figure 1.
Properties of T220/248. (A) Amino acid sequence of the peptide from the TSWV N protein in comparison to homologous sequences of other tospovirus N proteins. (B) Interactions between GusT peptide fusion and GUS, respectively, and C-terminal deletions of different tospovirus N proteins. Interactions were investigated in the yeast two-hybrid system by plating double-transformed yeast cells on SD-LW medium to test for double transformation and on SD-LWH medium for protein interactions. As a second reporter of the interaction, β-galactosidase was detected by using a filter lift assay. (C) Relative interaction strength is measured by a quantitative β-galactosidase activity assay. Yeast cells transformed with the empty vectors pAS2-1 and pACT2 were used as negative control (no column), whereas yeast cells expressing the CΔ26 protein interacting with TSWV N protein were the positive control. GUS protein did not interact with C-terminal deletions from N proteins of different origin.