Abstract
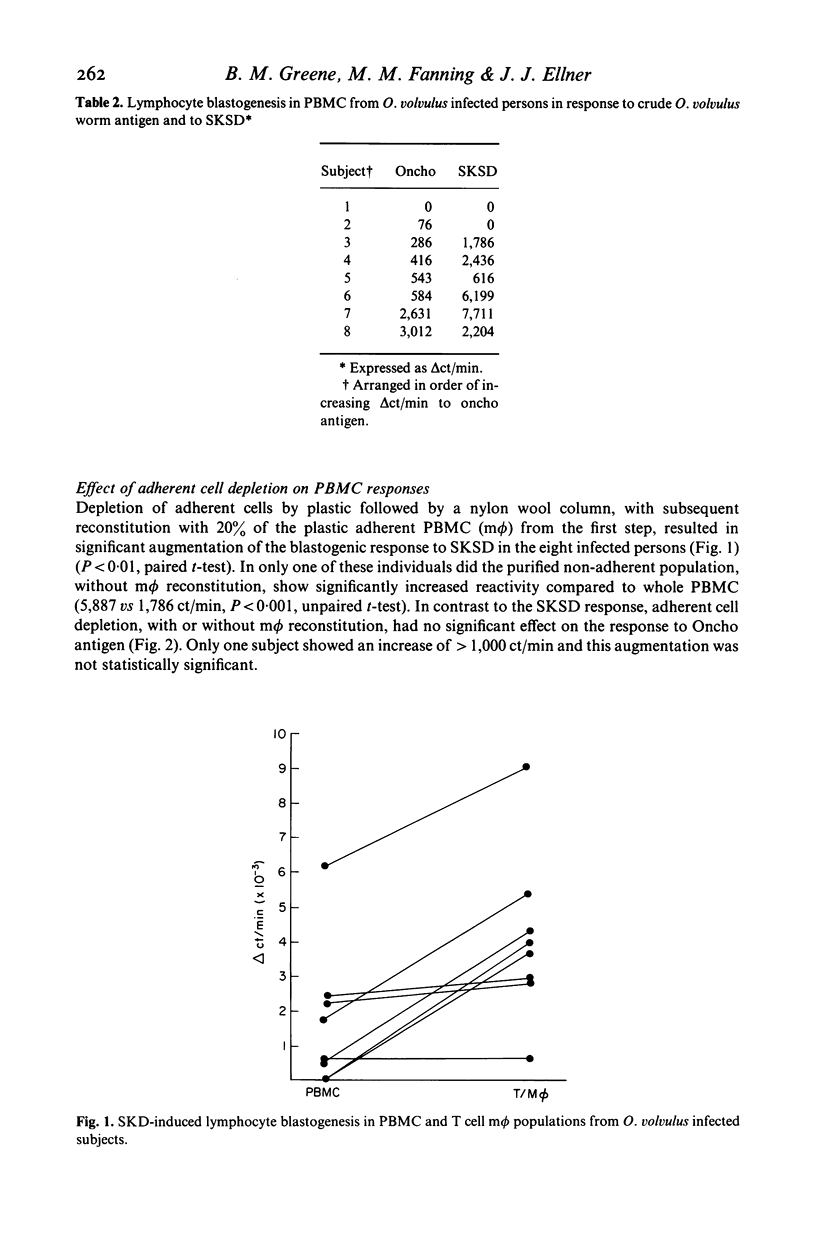
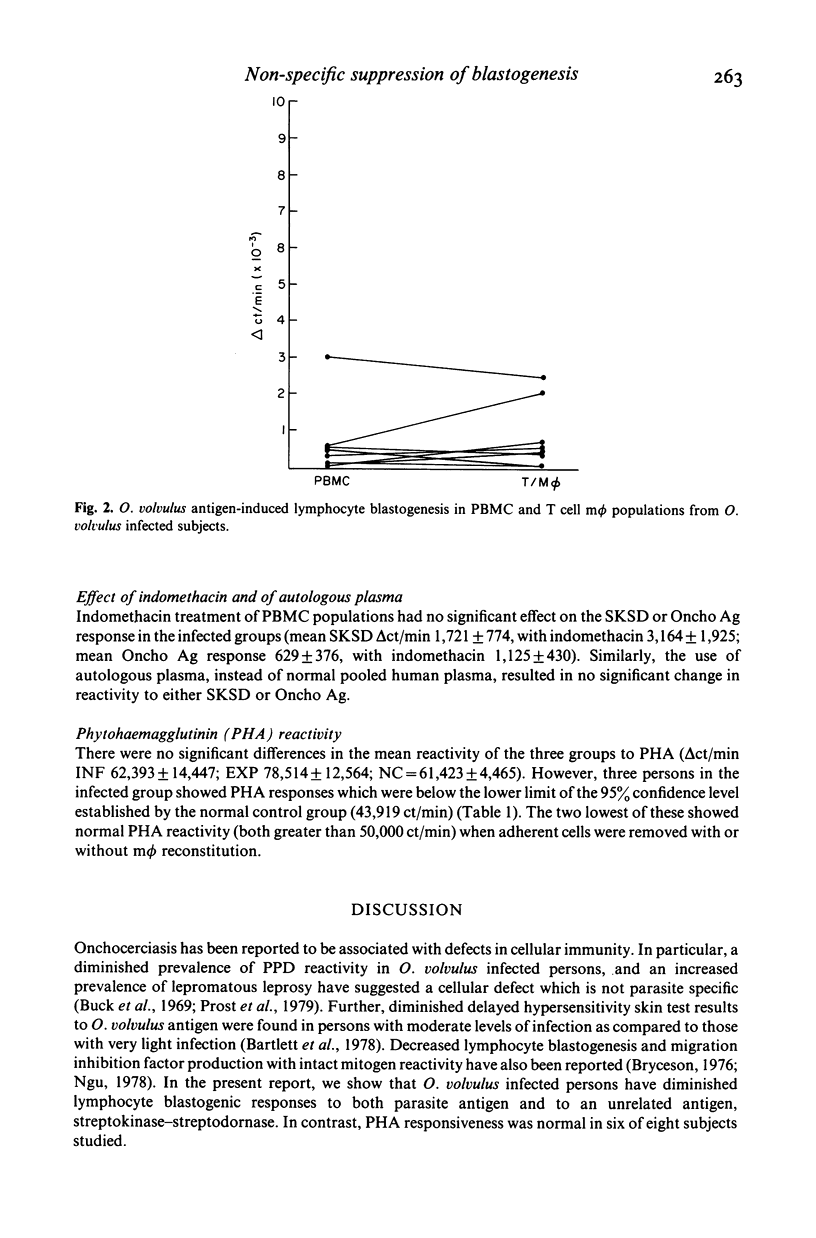
Lymphocyte blastogenic responses to O. volvulus antigen (Oncho Ag), SKSD, and the mitogen PHA were tested in three groups of persons: light to moderately infected persons (INF); previously exposed but uninfected persons (EXP) and normal controls (NC). The exposed group showed significant responsiveness to Oncho Ag (delta ct/min = 6,002 +/- 1,375), while the infected (delta ct/min = 943 +/- 418) and normal control (delta ct/min = 428 +/- 418) groups did not. The mean blastogenic response to SKSD were EXP, 8,644 +/- 5,249; NC 6,039 +/- 2,880; INF, 2,619 +/- 1,012. The reduced reactivity in the INF group to Oncho Ag showed a significant correlation with reactivity to SKSD (P less than 0.05). To elucidate the mechanism of hyporesponsiveness in the infected group rigorous adherent cell depletion, by adherence to plastic followed by a nylon wool column, was utilized. When 20% plastic adherent cells were added back to the T cells prepared in this fashion, the mean blastogenic response to SKSD was significantly augmented (P less than 0.01). In contrast, the responsiveness to Oncho Ag was not significantly altered. The addition of indomethacin (1 microgram/ml) or autologous plasma had no significant effect on reactivity to either SKSD or Oncho Ag. There were no significant differences in the mean reactivity of the three groups to PHA-M (delta ct/min EXP 78,514 +/- 12,564; INF 62,393 +/- 14,447; NC 61,423 +/- 4,465). These results suggest that O. volvulus infection is associated with decreased lymphocyte reactivity to both parasite related and unrelated antigens, and imply that the mechanism for the two types of hyporesponsiveness may be distinct. While a weakly adherent suppressor cell may account for non-specific hyporesponsiveness, the mechanism of parasite specific decreased reactivity remains unknown.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett A., Turk J., Ngu J., Mackenzie C. D., Fuglsang H. Variation in delayed hypersensitivity in onchocerciasis. Trans R Soc Trop Med Hyg. 1978;72(4):372–377. doi: 10.1016/0035-9203(78)90130-x. [DOI] [PubMed] [Google Scholar]
- Bryceson A. D. What happens when microfilariae die? Trans R Soc Trop Med Hyg. 1976;70(5-6):397–401. doi: 10.1016/0035-9203(76)90117-6. [DOI] [PubMed] [Google Scholar]
- Buck A. A., Anderson R. I., Kawata K., Hitchcock J. C., Jr Onchocerciasis: some new epidemiologic and clinical findings. Results of an epidemiologic study in the Republic of Chad. Am J Trop Med Hyg. 1969 Mar;18(2):217–230. doi: 10.4269/ajtmh.1969.18.217. [DOI] [PubMed] [Google Scholar]
- Ellner J. J. Suppressor adherent cells in human tuberculosis. J Immunol. 1978 Dec;121(6):2573–2579. [PubMed] [Google Scholar]
- Goodwin J. S., Bankhurst A. D., Messner R. P. Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J Exp Med. 1977 Dec 1;146(6):1719–1734. doi: 10.1084/jem.146.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene B. M., Taylor H. R., Aikawa M. Cellular killing of microfilariae of Onchocerca volvulus: eosinophil and neutrophil-mediated immune serum-dependent destruction. J Immunol. 1981 Oct;127(4):1611–1618. [PubMed] [Google Scholar]
- Henson P. M., Mackenzie C. D., Spector W. G. Inflammatory reactions in onchocerciasis: a report on current knowledge and recommendations for further study. Bull World Health Organ. 1979;57(5):667–682. [PMC free article] [PubMed] [Google Scholar]
- Miyama-Inaba M., Suzuki T., Paku Y. H., Masuda T. Feedback regulation of immune responses by immune complexes; possible involvement of a suppressive lymphokine by FcR gamma-bearing B cell. J Immunol. 1982 Feb;128(2):882–887. [PubMed] [Google Scholar]
- Ngu J. L. Immunological studies on onchocerciasis. Varying skin hypersensitivity and leucocyte migration inhibition in a clinical spectrum of the disease. Acta Trop. 1978 Sep;35(3):269–279. [PubMed] [Google Scholar]
- Ottesen E. A., Weller P. F., Heck L. Specific cellular immune unresponsiveness in human filariasis. Immunology. 1977 Sep;33(3):413–421. [PMC free article] [PubMed] [Google Scholar]
- Paganelli R., Ngu J. L., Levinsky R. J. Circulating immune complexes in onchocerciasis. Clin Exp Immunol. 1980 Mar;39(3):570–575. [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Piessens W. F., McGreevy P. B., Piessens P. W., McGreevy M., Koiman I., Saroso J. S., Dennis D. T. Immune responses in human infections with Brugia malayi: specific cellular unresponsiveness to filarial antigens. J Clin Invest. 1980 Jan;65(1):172–179. doi: 10.1172/JCI109648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piessens W. F., Partono F., Hoffman S. L., Ratiwayanto S., Piessens P. W., Palmieri J. R., Koiman I., Dennis D. T., Carney W. P. Antigen-specific suppressor T lymphocytes in human lymphatic filariasis. N Engl J Med. 1982 Jul 15;307(3):144–148. doi: 10.1056/NEJM198207153070302. [DOI] [PubMed] [Google Scholar]
- Piessens W. F., Ratiwayanto S., Tuti S., Palmieri J. H., Piessens P. W., Koiman I., Dennis D. T. Antigen-specific suppressor cells and suppressor factors in human filariasis with Brugia malayi. N Engl J Med. 1980 Apr 10;302(15):833–837. doi: 10.1056/NEJM198004103021503. [DOI] [PubMed] [Google Scholar]
- Rougemont A., Boisson-Pontal M. E., Pontal P. G., Gridel F., Sangare S. Tuberculin skin tests and B.C.G. vaccination in hyperendemic area of onchocerciasis. Lancet. 1977 Feb 5;1(8006):309–309. doi: 10.1016/s0140-6736(77)91857-8. [DOI] [PubMed] [Google Scholar]
- Steward M. W., Sisley B., Mackenzie C. D., El Sheikh H. Circulating antigen-antibody complexes in onchocerciasis. Clin Exp Immunol. 1982 Apr;48(1):17–24. [PMC free article] [PubMed] [Google Scholar]
- Yu A., Watts H., Jaffe N., Parkman R. Concomitant presence of tumor-specific cytotoxic and inhibitor lymphocytes in patients with osteogenic sarcoma. N Engl J Med. 1977 Jul 21;297(3):121–127. doi: 10.1056/NEJM197707212970301. [DOI] [PubMed] [Google Scholar]


