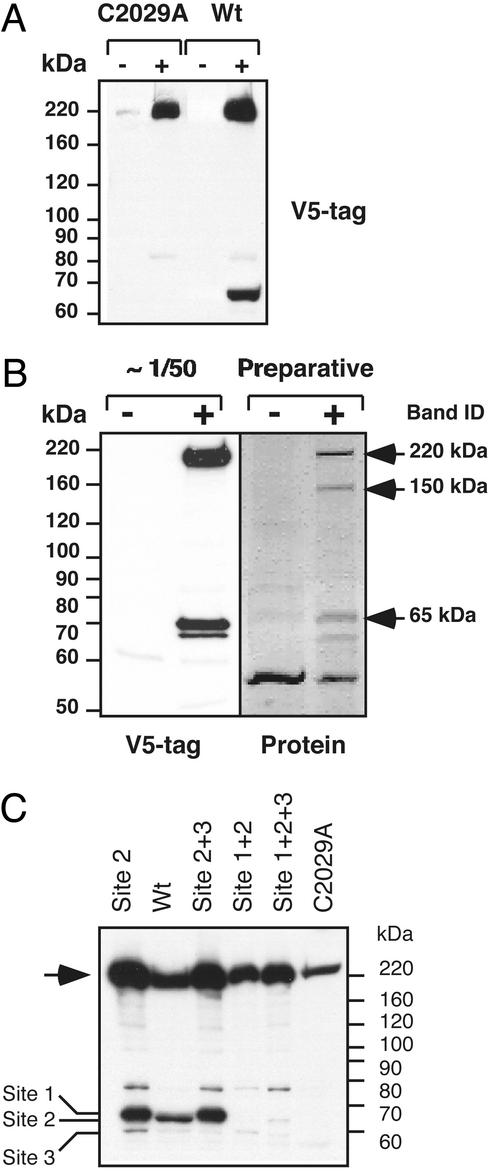
Figure 1.
Autocatalytic activity of separase at multiple sites is required for posttranslational processing of the enzyme. (A) Addition of ponasterone-induced (+) expression of V5-epitope C-terminally tagged C2029A mutant separase or wild-type (wt) separase in 293 cells. Uninduced cells (−) were used as a control. The C2029A mutant appears as a single polypeptide band corresponding to the full-length molecule. Expression of the wild-type separase results in appearance of a 65-kDa cleavage product. The membrane was probed with V5 antibodies. (B) Identification of the major cleavage site in human separase. Expression of V5-epitope tagged wild-type separase was induced for 14 h and immunoprecipitated with V5-tag antibodies. Ninety-five percent of the precipitated proteins were separated by SDS/PAGE, visualized by Ponceau S staining (Preparative), or used for Western blotting with V5 antibodies. N-terminal sequencing analysis was performed on the 220-, 150-, and 65-kDa protein bands, indicated by the arrows. Sequencing results are given in the text. + and −, induced and noninduced cells, respectively. (C) C-terminally epitope-tagged separase and mutant derivatives were transiently expressed in HeLa cells, and the whole-cell extracts were analyzed by Western blot with V5 antibodies (see text for the description of the mutants). Full-length separase (arrow) and the proteolytic fragments resulting from cleavage at each of the three sites are indicated (left).