Abstract
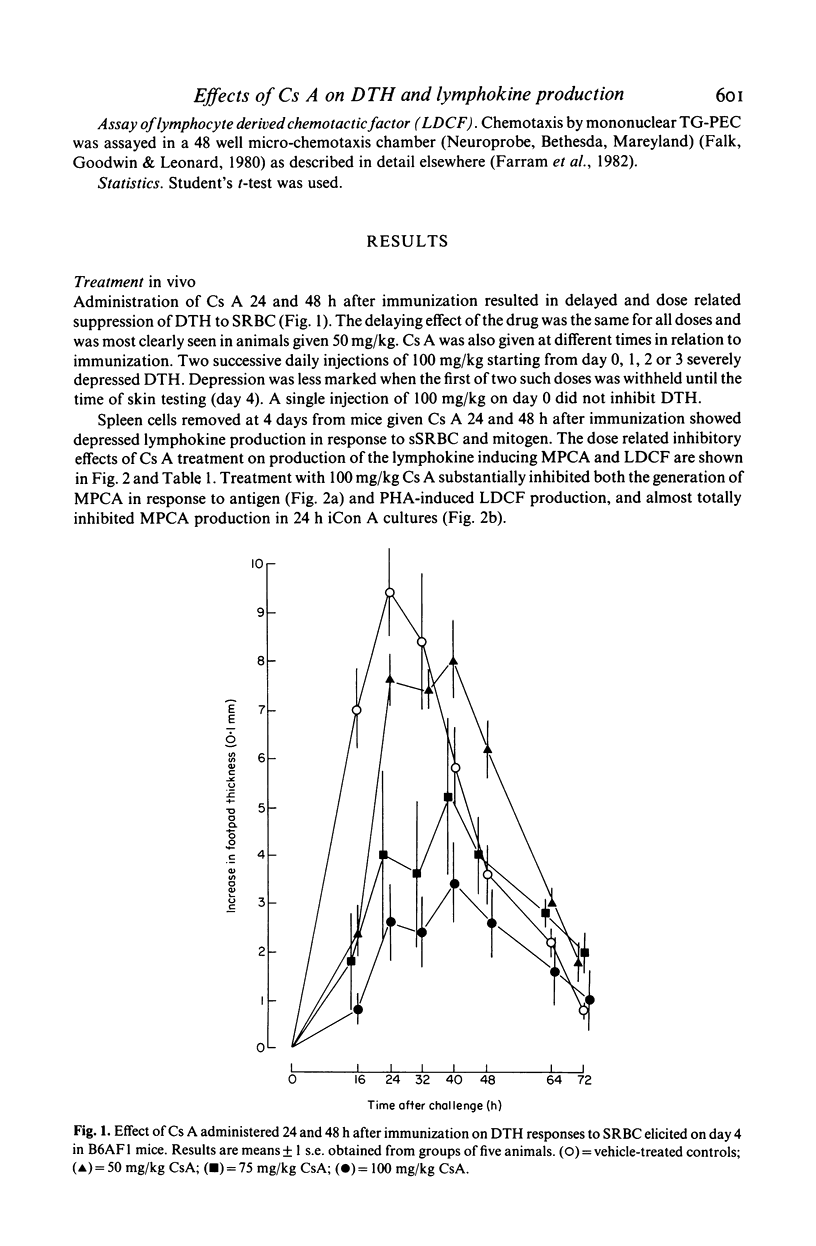
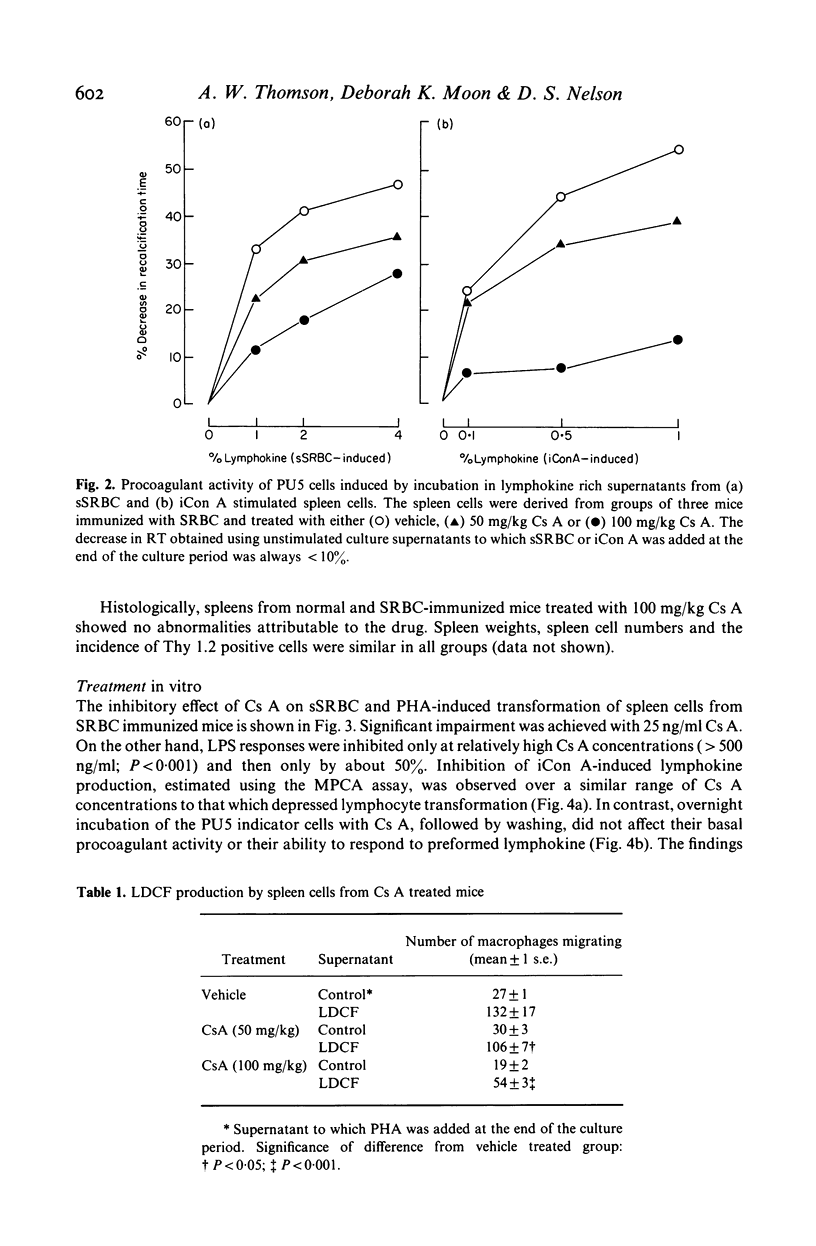
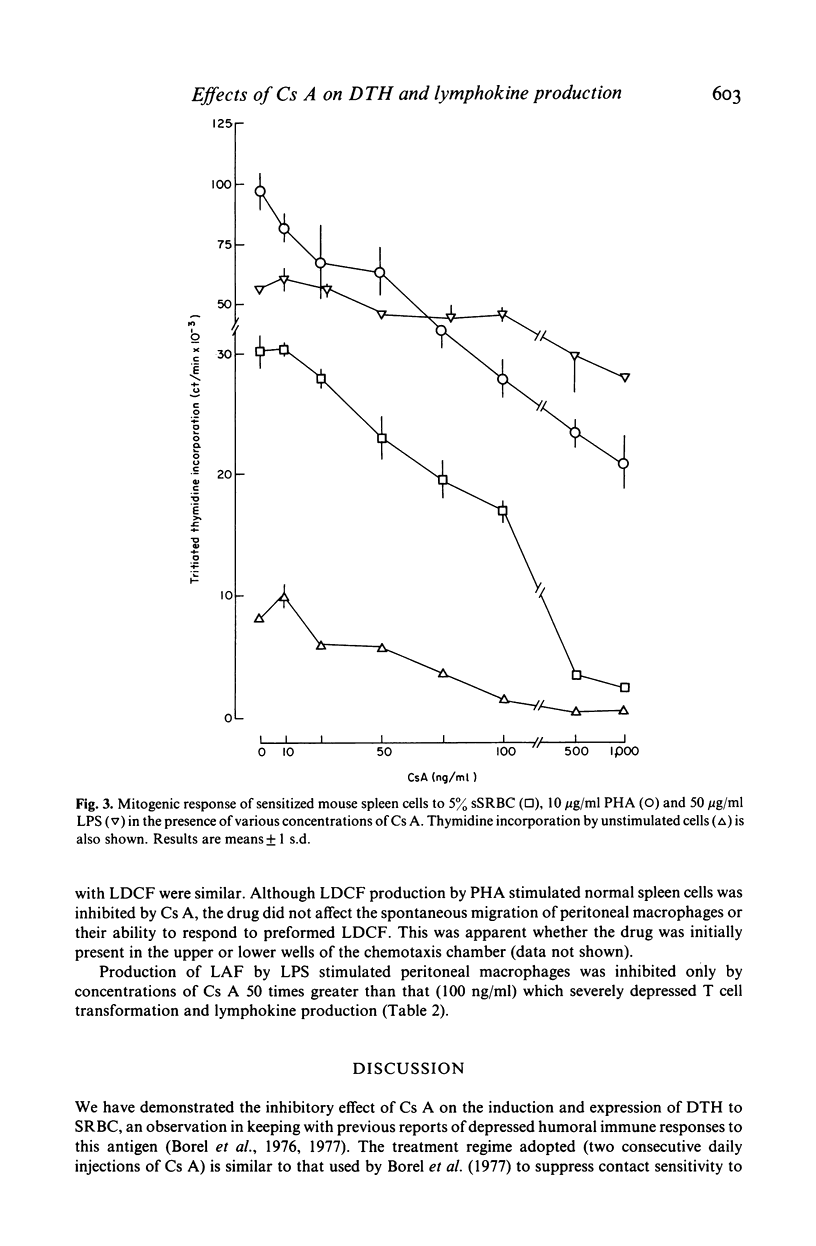
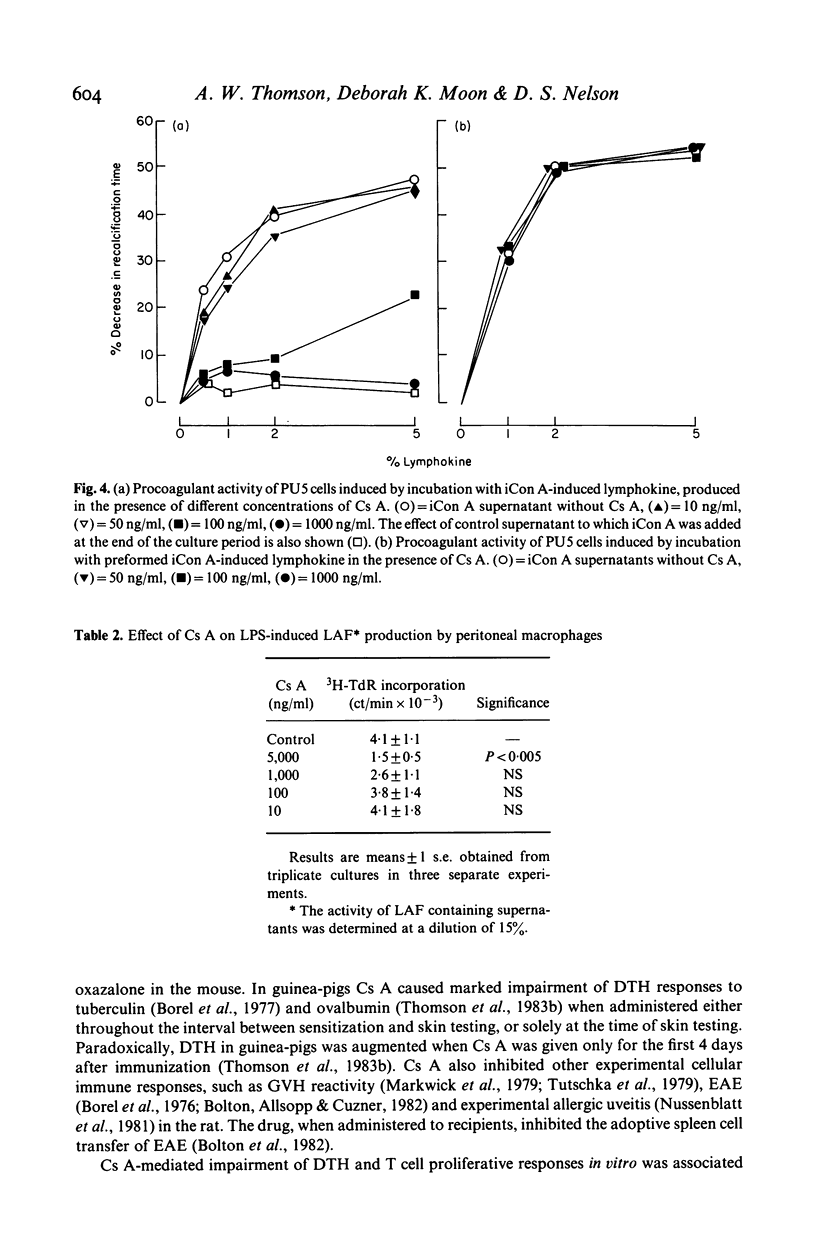
Two consecutive daily i.m. injections of cyclosporin A (Cs A) (greater than 50 mg/kg) inhibited delayed type hypersensitivity (DTH) responses in mice immunized with SRBC. Maximal suppression was observed when Cs A was administered 24 and 48 h after sensitization. Culture of spleen cells from these animals with antigen, insoluble concanavalin A (iCon A) or PHA revealed inhibition of the production of two lymphokines: that inducing macrophage procoagulant activity (MPCA) and macrophage chemotactic factor (LDCF). The inhibitory effect on lymphokine production was not due to depletion of T cells. In vitro, 25 ng/ml Cs A suppressed T cell proliferative responses to antigen and mitogen but much higher doses were required to impair the response to LPS. Similar doses of Cs A also suppressed lymphokine production, but the responses of macrophages to these lymphokines was unaffected, even at doses which totally inhibited lymphokine production. Production of interleukin 1 by LPS stimulated macrophages was inhibited by Cs A only at concentrations much greater than those required to suppress lymphokine production.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberti S., Boraschi D., Luini W., Tagliabue A. Effects of in vivo treatments with cyclosporin-A on mouse cell-mediated immune responses. Int J Immunopharmacol. 1981;3(4):357–364. doi: 10.1016/0192-0561(81)90031-x. [DOI] [PubMed] [Google Scholar]
- Andrus L., Lafferty K. J. Inhibition of T-cell activity by cyclosporin A. Scand J Immunol. 1981 May;15(5):449–458. doi: 10.1111/j.1365-3083.1982.tb00670.x. [DOI] [PubMed] [Google Scholar]
- Bolton C., Allsopp G., Cuzner M. L. The effect of cyclosporin A on the adoptive transfer of experimental allergic encephalomyelitis in the Lewis rat. Clin Exp Immunol. 1982 Jan;47(1):127–132. [PMC free article] [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Magnée C., Stähelin H. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology. 1977 Jun;32(6):1017–1025. [PMC free article] [PubMed] [Google Scholar]
- Britton S., Palacios R. Cyclosporin A--usefulness, risks and mechanism of action. Immunol Rev. 1982;65:5–22. doi: 10.1111/j.1600-065x.1982.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Bunjes D., Hardt C., Röllinghoff M., Wagner H. Cyclosporin A mediates immunosuppression of primary cytotoxic T cell responses by impairing the release of interleukin 1 and interleukin 2. Eur J Immunol. 1981 Aug;11(8):657–661. doi: 10.1002/eji.1830110812. [DOI] [PubMed] [Google Scholar]
- Burckhardt J. J., Guggenheim B. Cyclosporin A: in vivo and in vitro suppression of rat T-lymphocyte function. Immunology. 1979 Apr;36(4):753–757. [PMC free article] [PubMed] [Google Scholar]
- Calne R. Y., White D. J., Evans D. B., Thiru S., Henderson R. G., Hamilton D. V., Rolles K., McMaster P., Duffy T. J., MacDougall B. R. Cyclosporin A in cadaveric organ transplantation. Br Med J (Clin Res Ed) 1981 Mar 21;282(6268):934–936. doi: 10.1136/bmj.282.6268.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calne R. Y., White D. J., Thiru S., Evans D. B., McMaster P., Dunn D. C., Craddock G. N., Pentlow B. D., Rolles K. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet. 1978 Dec 23;2(8104-5):1323–1327. doi: 10.1016/s0140-6736(78)91970-0. [DOI] [PubMed] [Google Scholar]
- Colvin R. B., Johnson R. A., Mihm M. C., Jr, Dvorak H. F. Role of the clotting system in cell-mediated hypersensitivity. I. Fibrin deposition in delayed skin reactions in man. J Exp Med. 1973 Sep 1;138(3):686–698. doi: 10.1084/jem.138.3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Toit D. F., Reece-Smith H., McShane P., Denton T., Morris P. J. Prolongation of segmental pancreatic allografts in dogs receiving cyclosporin A. Transplantation. 1982 Apr;33(4):432–437. [PubMed] [Google Scholar]
- Dunn D. C., White D. J., Herbertson B. M., Wade J. Prolongation of kidney survival during and after cyclosporin A therapy. Transplantation. 1979 May;27(5):359–361. doi: 10.1097/00007890-197905000-00018. [DOI] [PubMed] [Google Scholar]
- Falk W., Goodwin R. H., Jr, Leonard E. J. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33(3):239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- Farram E., Nelson M., Nelson D. S., Moon D. K. Inhibition of cytokine production by a tumor cell product. Immunology. 1982 Jul;46(3):603–612. [PMC free article] [PubMed] [Google Scholar]
- Geczy C. L., Meyer P. A. Leukocyte procoagulant activity in man: an in vitro correlate of delayed-type hypersensitivity. J Immunol. 1982 Jan;128(1):331–336. [PubMed] [Google Scholar]
- Gordon M. Y., Singer J. W. Selective effects of cyclosporin A on colony-forming lymphoid and myeloid cells in man. Nature. 1979 May 31;279(5712):433–434. doi: 10.1038/279433a0. [DOI] [PubMed] [Google Scholar]
- Green C. J., Allison A. C. Extensive prolongation of rabbit kidney allograft survival after short-term cyclosporin-A treatment. Lancet. 1978 Jun 3;1(8075):1182–1183. doi: 10.1016/s0140-6736(78)90970-4. [DOI] [PubMed] [Google Scholar]
- Hess A. D., Tutschka P. J. Effect of cyclosporin A on human lymphocyte responses in vitro. I. CsA allows for the expression of alloantigen-activated suppressor cells while preferentially inhibiting the induction of cytolytic effector lymphocytes in MLR. J Immunol. 1980 Jun;124(6):2601–2608. [PubMed] [Google Scholar]
- Hess A. D., Tutschka P. J., Santos G. W. Effect of cyclosporin A on human lymphocyte responses in vitro. III. CsA inhibits the production of T lymphocyte growth factors in secondary mixed lymphocyte responses but does not inhibit the response of primed lymphocytes to TCGF. J Immunol. 1982 Jan;128(1):355–359. [PubMed] [Google Scholar]
- Homan W. P., Fabre J. W., Morris P. J. Nature of the unresponsiveness induced by cyclosporin A in rats bearing renal allografts. Transplantation. 1979 Nov;28(5):439–441. doi: 10.1097/00007890-197911000-00023. [DOI] [PubMed] [Google Scholar]
- Homan W. P., Fabre J. W., Williams K. A., Millard P. R., Morris P. J. Studies on the immunosuppressive properties of cyclosporin a in rats receiving renal allografts. Transplantation. 1980 May;29(5):361–366. doi: 10.1097/00007890-198005000-00003. [DOI] [PubMed] [Google Scholar]
- Hopper K. E., Geczy C. L., Davies W. A. A mechanism of migration inhibition in delayed-type hypersensitivity reactions. I. Fibrin deposition on the surface of elicited peritoneal macrophages on vivo. J Immunol. 1981 Mar;126(3):1052–1058. [PubMed] [Google Scholar]
- Markwick J. R., Chambers J. D., Hobbs J. R., Pegrum G. D. Timing of cyclosporin-A therapy for abrogation of HVG and GVH responses in rats. Lancet. 1979 Nov 17;2(8151):1037–1040. doi: 10.1016/s0140-6736(79)92441-3. [DOI] [PubMed] [Google Scholar]
- McIntosh L. C., Thompson A. W. Activity of the mononuclear phagocyte system in cyclosporin A-treated mice. Transplantation. 1980 Nov;30(5):384–386. doi: 10.1097/00007890-198011000-00017. [DOI] [PubMed] [Google Scholar]
- Nelson D. S. The effects of anticoagulants and other drugs on cellular and cutaneous reactions to antigen in guinea-pigs with delayed-type hypersensitivity. Immunology. 1965 Sep;9(3):219–234. [PMC free article] [PubMed] [Google Scholar]
- Nelson M., Nelson D. S. Macrophages and resistance to tumours. I. Inhibition of delayed-type hypersensitivity reactions by tumour cells and by soluble prducts affecting macrophages. Immunology. 1978 Feb;34(2):277–290. [PMC free article] [PubMed] [Google Scholar]
- Nussenblatt R. B., Rodrigues M. M., Wacker W. B., Cevario S. J., Salinas-Carmona M. C., Gery I. Cyclosporin a. Inhibition of experimental autoimmune uveitis in Lewis rats. J Clin Invest. 1981 Apr;67(4):1228–1231. doi: 10.1172/JCI110138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavonen T., Järveläinen H., Kontiainen S., Häyry P. Effect of cyclosporin A on in vitro proliferative activity and immunoglobulin synthesis of isolated human lymphoid cell subpopulations. Clin Exp Immunol. 1981 Feb;43(2):342–350. [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Möller G. Cyclosporin A blocks receptors for HLA-DR antigens on T cells. Nature. 1981 Apr 30;290(5809):792–794. doi: 10.1038/290792a0. [DOI] [PubMed] [Google Scholar]
- Starzl T. E., Klintmalm G. B., Weil R., 3rd, Porter K. A., Iwatsuki S., Schroter G. P., Fernandez-Bueno C., MacHugh N. Cyclosporin A and steroid therapy in sixty-six cadaver kidney recipients. Surg Gynecol Obstet. 1981 Oct;153(4):486–494. [PMC free article] [PubMed] [Google Scholar]
- Tutschka P. J., Beschorner W. E., Allison A. C., Burns W. H., Santos G. W. Use of cyclosporin A in allogeneic bone marrow transplantation in the rat. Nature. 1979 Jul 12;280(5718):148–151. doi: 10.1038/280148a0. [DOI] [PubMed] [Google Scholar]
- Waterston R. H. Soluble antigen from sheep erythrocytes: preparation and antigenic properties. Immunology. 1970 Mar;18(3):431–435. [PMC free article] [PubMed] [Google Scholar]
- White D. J., Plumb A. M., Pawelec G., Brons G. Cyclosporin A: an immunosuppressive agent preferentially active against proliferating T cells. Transplantation. 1979 Jan;27(1):55–58. [PubMed] [Google Scholar]


