Abstract
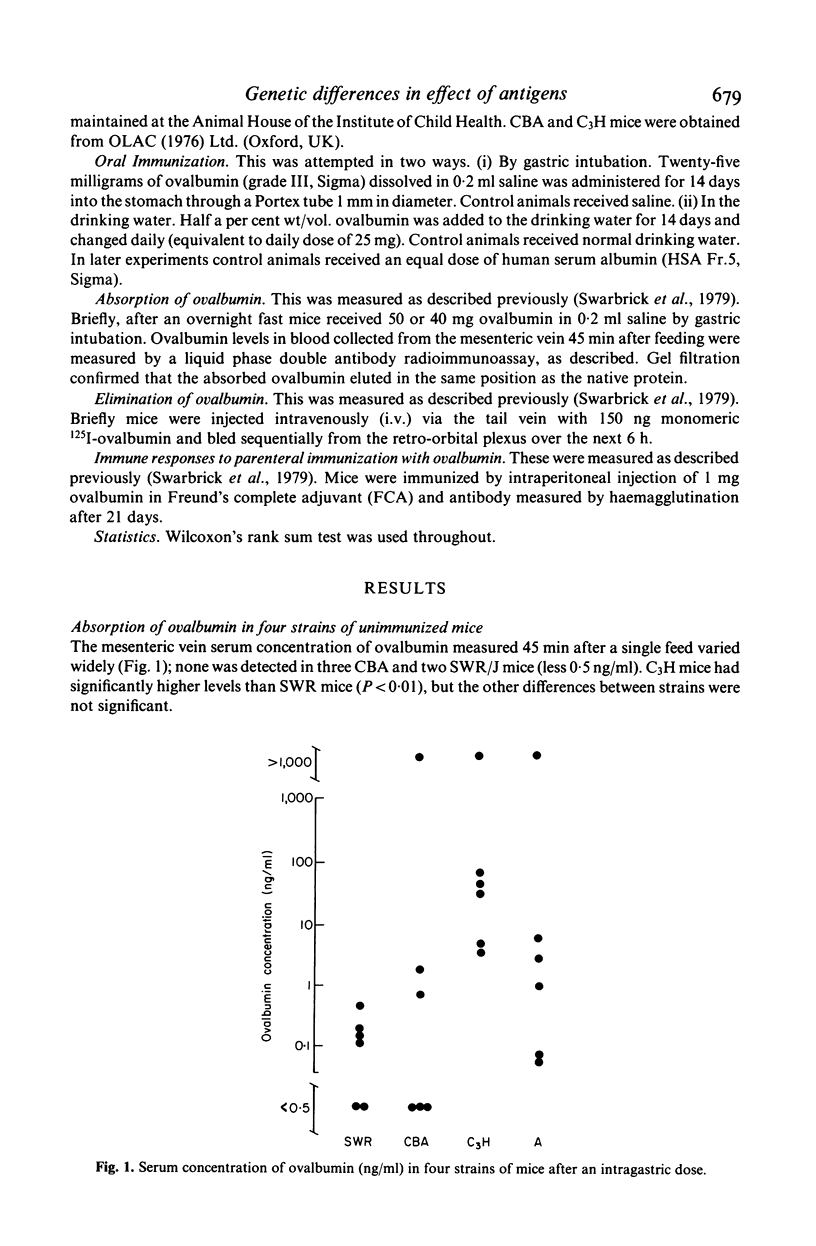
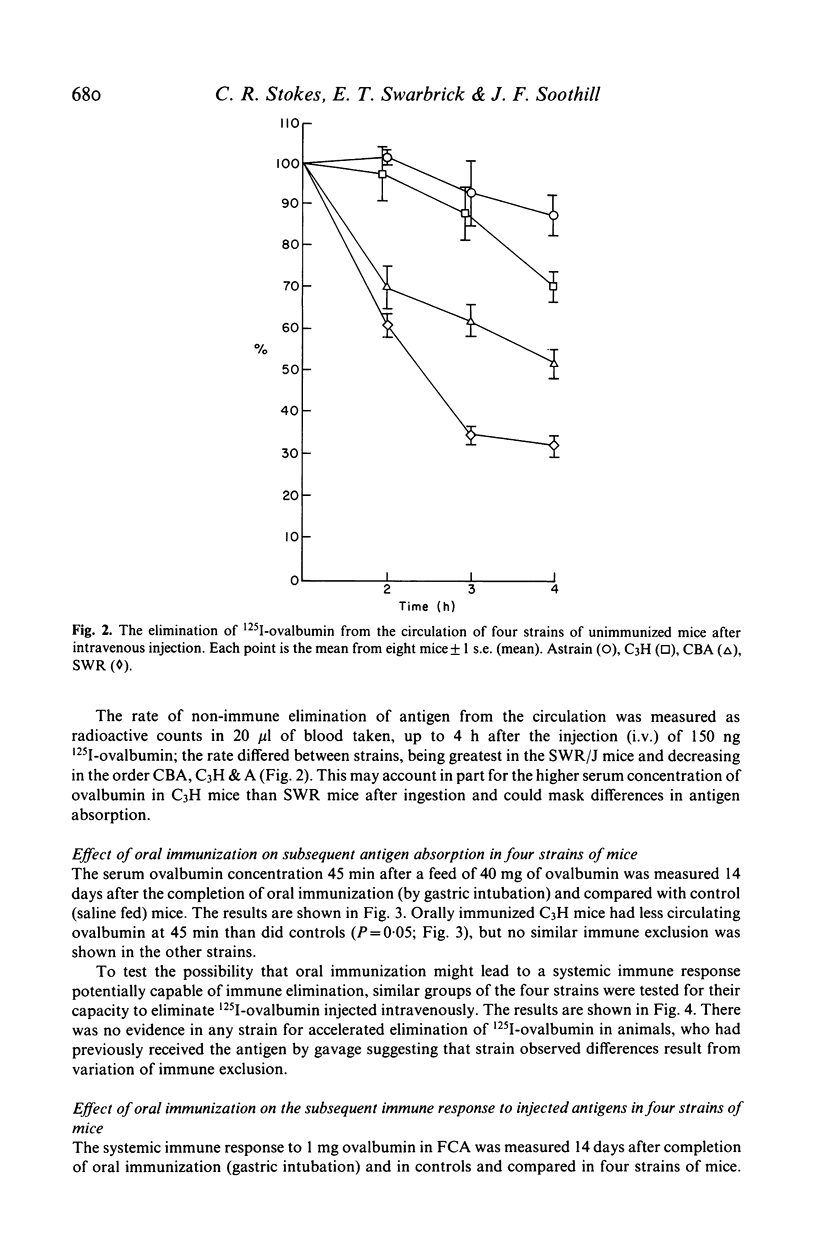
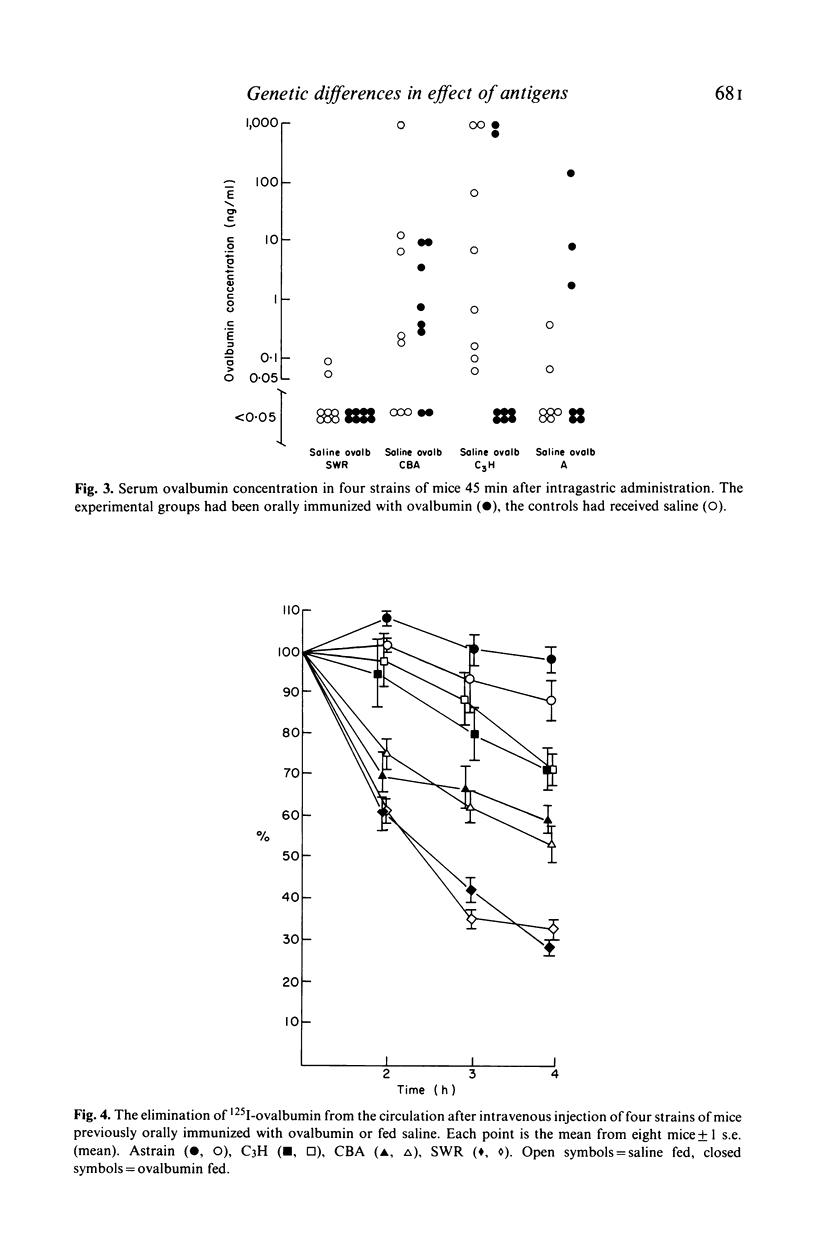
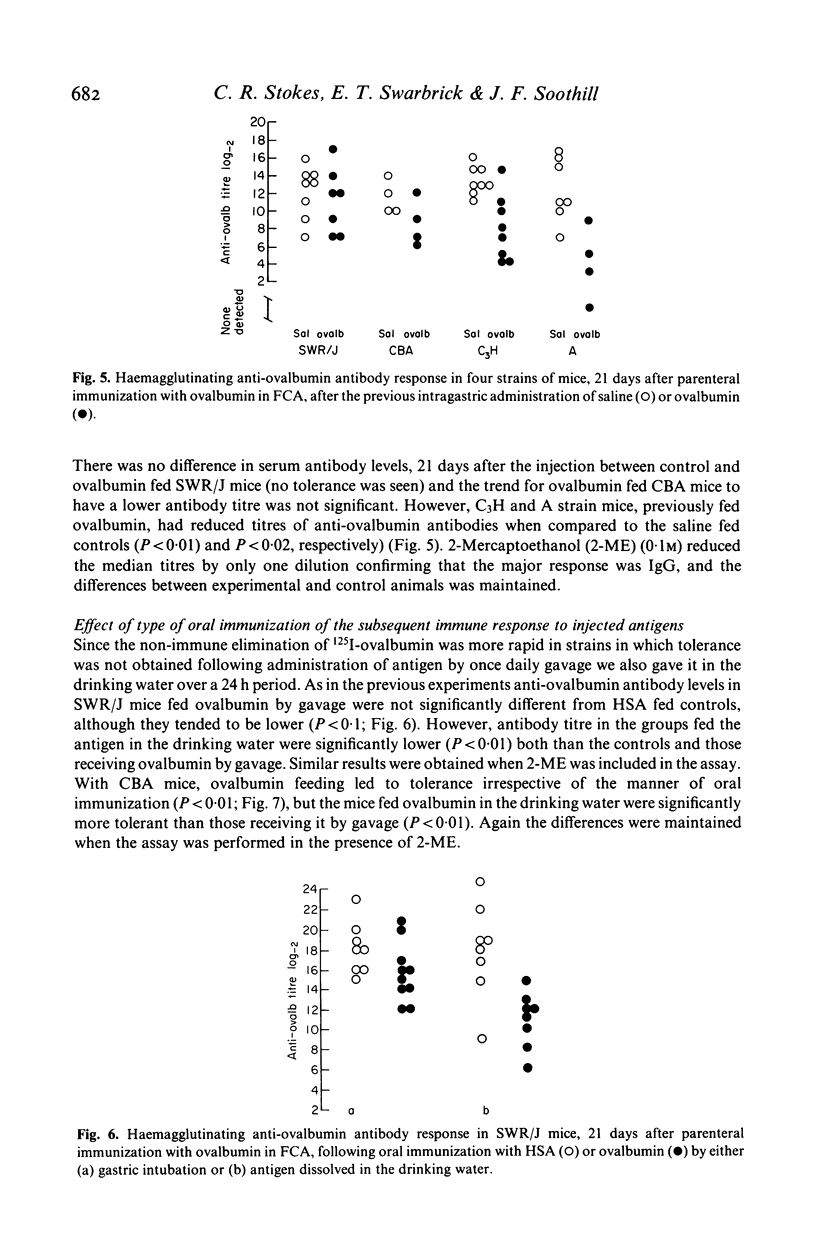
The effect of feeding ovalbumin was studied in CBA, C3H, SWR/J and A inbred strains of mice. In non-immune animals differences were observed in their abilities to absorb intact antigen and eliminate it from their circulation. Following oral immunization, C3H mice were able to absorb significantly less ovalbumin than controls, however no evidence for immune exclusion was found in the other strains. Oral tolerance could be induced in all strains tested but in CBA and SWR/J mice oral immunization had to be continual, via the drinking rather than a single daily gastric intubation. The results suggest that genetic differences exist in the handling of antigen by the gut. If similar differences exist in man they may underly differences in susceptibility to food allergy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers J. H., Steward M. W., Soothill J. F. Differences in immune elimination in inbred mice. The role of low affinity antibody. Clin Exp Immunol. 1972 Sep;12(1):121–132. [PMC free article] [PubMed] [Google Scholar]
- Bazin H., Platteau B. Oral feeding of ovalbumin can make rats tolerant to an intraperitoneal injection of dinitrophenylated ovalbumin and Bordetella pertussis vaccine [proceedings]. Biochem Soc Trans. 1977;5(5):1571–1573. doi: 10.1042/bst0051571. [DOI] [PubMed] [Google Scholar]
- Devey M. E., Steward M. W. The induction of chronic antigen-antibody complex disease in selectively bred mice producing either high or low affinity antibody to protein antigens. Immunology. 1980 Oct;41(2):303–311. [PMC free article] [PubMed] [Google Scholar]
- Hanson D. G., Vaz N. M., Maia L. C., Hornbrook M. M., Lynch J. M., Roy C. A. Inhibition of specific immune responses by feeding protein antigens. Int Arch Allergy Appl Immunol. 1977;55(1-6):526–532. doi: 10.1159/000231966. [DOI] [PubMed] [Google Scholar]
- Jarrett E. E., Haig D. M., McDougall W., McNulty E. Rat IgE production. II. Primary and booster reaginic antibody responses following intradermal or oral immunization. Immunology. 1976 May;30(5):671–677. [PMC free article] [PubMed] [Google Scholar]
- Jarrett E. E., Stewart D. C. Rat IgE production. I. Effect of dose of antigen on primary and secondary reaginic antibody responses. Immunology. 1974 Sep;27(3):365–381. [PMC free article] [PubMed] [Google Scholar]
- MOTA I. THE MECHANISM OF ANAPHYLAXIS. I. PRODUCTION AND BIOLOGICAL PROPERTIES OF 'MAST CELL SENSITIZING' ANTIBODY. Immunology. 1964 Nov;7:681–699. [PMC free article] [PubMed] [Google Scholar]
- Morgan A. G., Soothill J. F. Relationship between macrophage clearance of PVP and affinity of anti-protein antibody response in inbred mouse strains. Nature. 1975 Apr 24;254(5502):711–712. doi: 10.1038/254711a0. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., Ferguson A. Hypersensitivity in the small intestinal mucosa. V. Induction of cell-mediated immunity to a dietary antigen. Clin Exp Immunol. 1981 Mar;43(3):574–582. [PMC free article] [PubMed] [Google Scholar]
- Newby T. J., Stokes C. R., Bourne F. J. Altered polyvinylpyrrolidone clearance and immune responsiveness caused by small dietary changes. Clin Exp Immunol. 1980 Feb;39(2):349–354. [PMC free article] [PubMed] [Google Scholar]
- Newby T. J., Stokes C. R., Bourne F. J. Effects of feeding bacterial lipopolysaccharide and dextran sulphate on the development of oral tolerance to contact sensitizing agents. Immunology. 1980 Nov;41(3):617–621. [PMC free article] [PubMed] [Google Scholar]
- Passwell J. H., Steward M. W., Soothill J. F. The effects of protein malnutrition on macrophage function and the amount and affinity of antibody response. Clin Exp Immunol. 1974 Jul;17(3):491–495. [PMC free article] [PubMed] [Google Scholar]
- Perrudet-Badoux A., Binaghi R. A., Biozzi G. Trichinella infestation in mice genetically selected for high and low antibody production. Immunology. 1975 Aug;29(2):387–390. [PMC free article] [PubMed] [Google Scholar]
- Petty R. E., Steward M. W., Soothill J. F. The heterogeneity of antibody affinity in inbred mice and its possible immunopathologic significance. Clin Exp Immunol. 1972 Oct;12(2):231–241. [PMC free article] [PubMed] [Google Scholar]
- Soothill J. F., Steward M. W. The immunopathological significance of the heterogeneity of antibody affinity. Clin Exp Immunol. 1971 Aug;9(2):193–199. [PMC free article] [PubMed] [Google Scholar]
- Swarbrick E. T., Stokes C. R., Soothill J. F. Absorption of antigens after oral immunisation and the simultaneous induction of specific systemic tolerance. Gut. 1979 Feb;20(2):121–125. doi: 10.1136/gut.20.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannemuehler M. J., Kiyono H., Babb J. L., Michalek S. M., McGhee J. R. Lipopolysaccharide (LPS) regulation of the immune response: LPS converts germfree mice to sensitivity to oral tolerance induction. J Immunol. 1982 Sep;129(3):959–965. [PubMed] [Google Scholar]


