Figure 1.
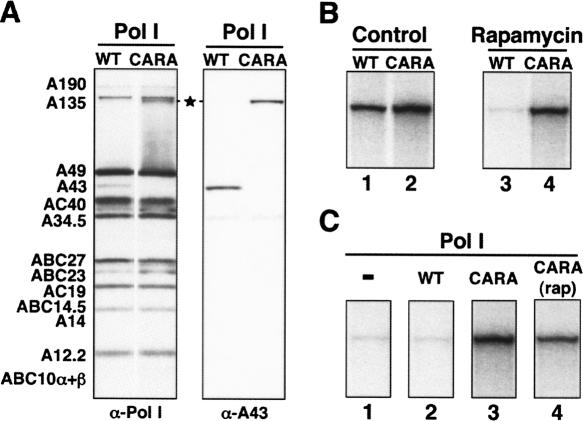
Pol I from CARA cells mimics a nondissociable Pol I-Rrn3 complex. (A) Western blot analysis of purified Pol I. The subunit composition of the same amount of Pol I purified from wild-type (WT) or CARA cells grown in rich medium to mid–log phase was analyzed by Western blot using anti-Pol I or anti-A43 antibodies. Both forms of enzyme display the same subunit composition, except that the A43 protein is missing in the Pol I purified from CARA cells and is replaced by the Rrn3-A43 fusion protein (★), which is stably and stoichiometrically assembled. (B) Repression of in vitro Pol I transcription by rapamycin. WT or CARA cells were grown in rich medium to mid–log phase, treated with rapamycin for 30 min (Rapamycin) or not (Control), and then harvested at the same optical density (OD600 = 1.5). Partially purified extracts (PA600) from WT (lanes 1,3) or CARA cells (lanes 2,4) containing the same amount of Pol I (see Supplemental Fig. S3), were assayed for in vitro Pol I-specific transcription using a mini-rDNA gene as template. Without rapamycin treatment, efficient Pol I transcription is observed in both WT and CARA extracts (lanes 1,2). When cells were treated with rapamycin, traces of Pol I transcription were detected in WT extract (lane 3), whereas CARA extract remained fully active (lane 4). (C) Reactivation of rapamycin-treated WT cell extract. Extracts prepared from rapamycin-treated WT cells, deficient for Pol I transcription (lane 1) were complemented by addition of 200 ng of Pol I purified either from untreated WT cells (lane 2), untreated CARA cells (lane 3), or rapamycin-treated CARA cells (lane 4). Enzyme prepared from untreated and rapamycin-treated CARA cells reactivates Pol I transcription of rapamycin-treated WT cell extract, whereas Pol I prepared from WT cells has no effect.