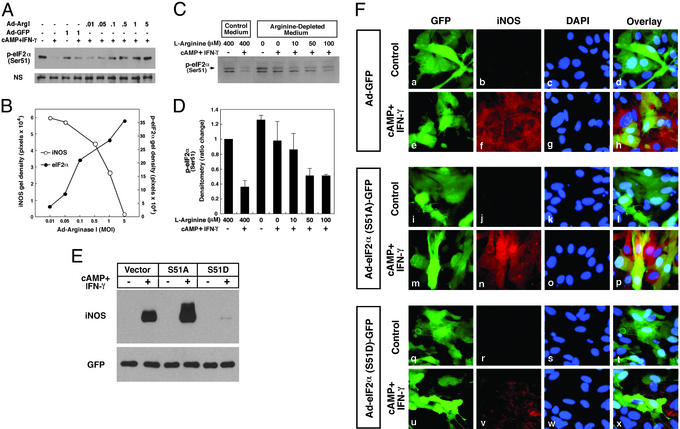
Figure 4.
Extracellular or intracellular manipulation of intracellular arginine decreases iNOS expression by translational control mechanisms involving eIF2α phosphorylation. (A) Arginase I-induced decreases in iNOS protein levels are associated with the phosphorylation of eIF2α. Rat astrocytes were infected with Ad-Arg I or Ad-GFP for 24 h. Cells were then stimulated by cAMP (1 mM) and IFN-γ (100 units/ml) for 16 h. Whole-cell lysates were subjected to immunoblotting with antibodies specific for the form of eIF2α that is phosphorylated at serine 51. (B) An inverse correlation is observed between the level of iNOS protein and the amount of phospho-eIF2α protein. The densitometric values were calculated from iNOS and phospho-eIF2α immunoblots shown in A and Fig. 2D. (C) Increased levels of extracellular arginine are correlated with a decrease in the level of intracellular phospho-eIF2α. (D) The densitometric value (mean ± SD) of phospho-eIF2α from three separate experiments. Rat astrocytes were cultured in l-arginine-depleted medium or l-arginine-supplemented medium for 24 h. Cells were then stimulated by cAMP (1 mM) and IFN-γ (100 units/ml) for 16 h. (E) Rat astrocytes were infected with Ad-GFP (vector control), Ad-S51A (serine to alanine, nonphosphorylatable mutant), or Ad-S51D (serine to aspartate, phosphomimetic mutant) at an moi of 5 for 24 h and subsequently treated with cAMP (1 mM) and IFN-γ (100 units/ml) for 16 h. Immunoblot analysis of iNOS in whole-cell lysates is shown. (F) For immunofluorescence microscopy, cells were fixed with 4% paraformaldehyde and incubated with rabbit anti-iNOS antibody (b, f, j, n, r, and v) or the nuclear stain 4′,6-diamidino-2-phenylindole (DAPI) (c, g, k, o, s, and w). The adenovirus transfer vector used in these studies has a double expression cassette, and GFP has been subcloned into Ad-GFP (a and e), Ad-S51A (i and m), or Ad-S51D (q and u) to monitor vector-derived expression.