Abstract
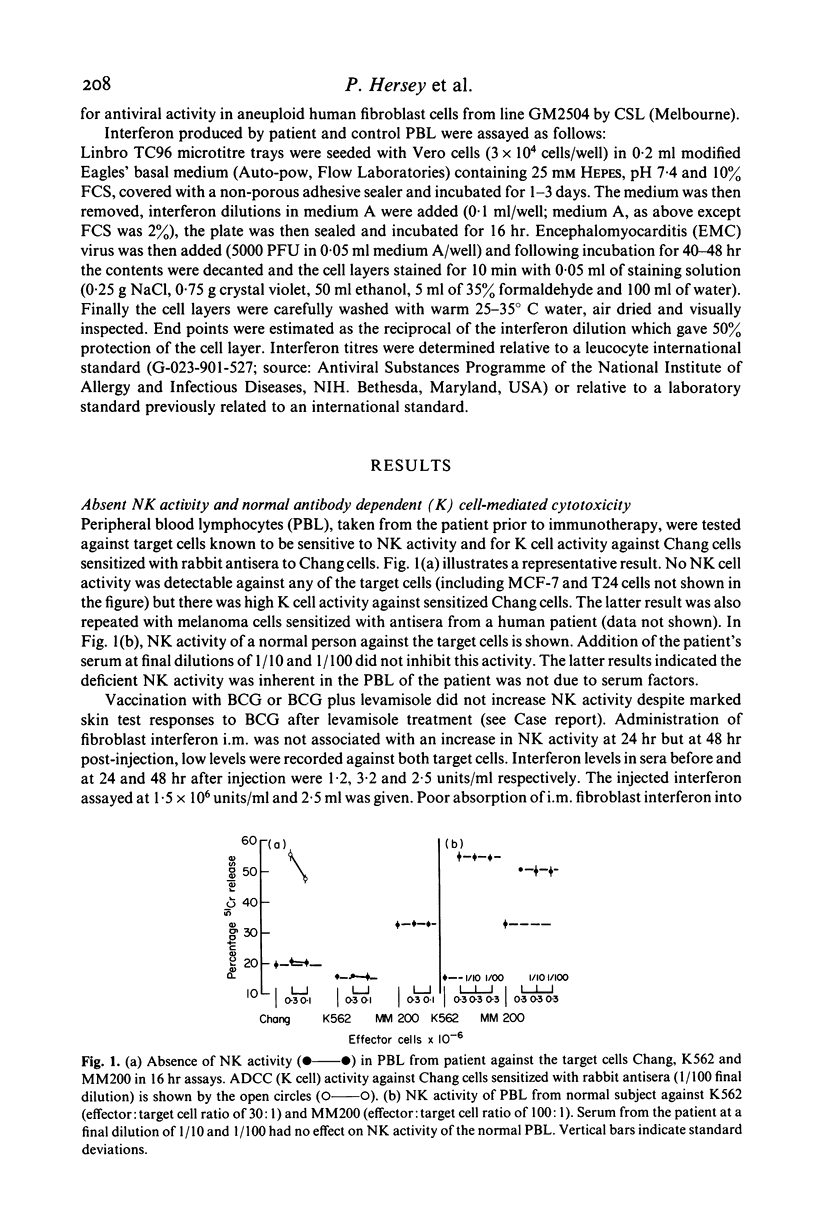
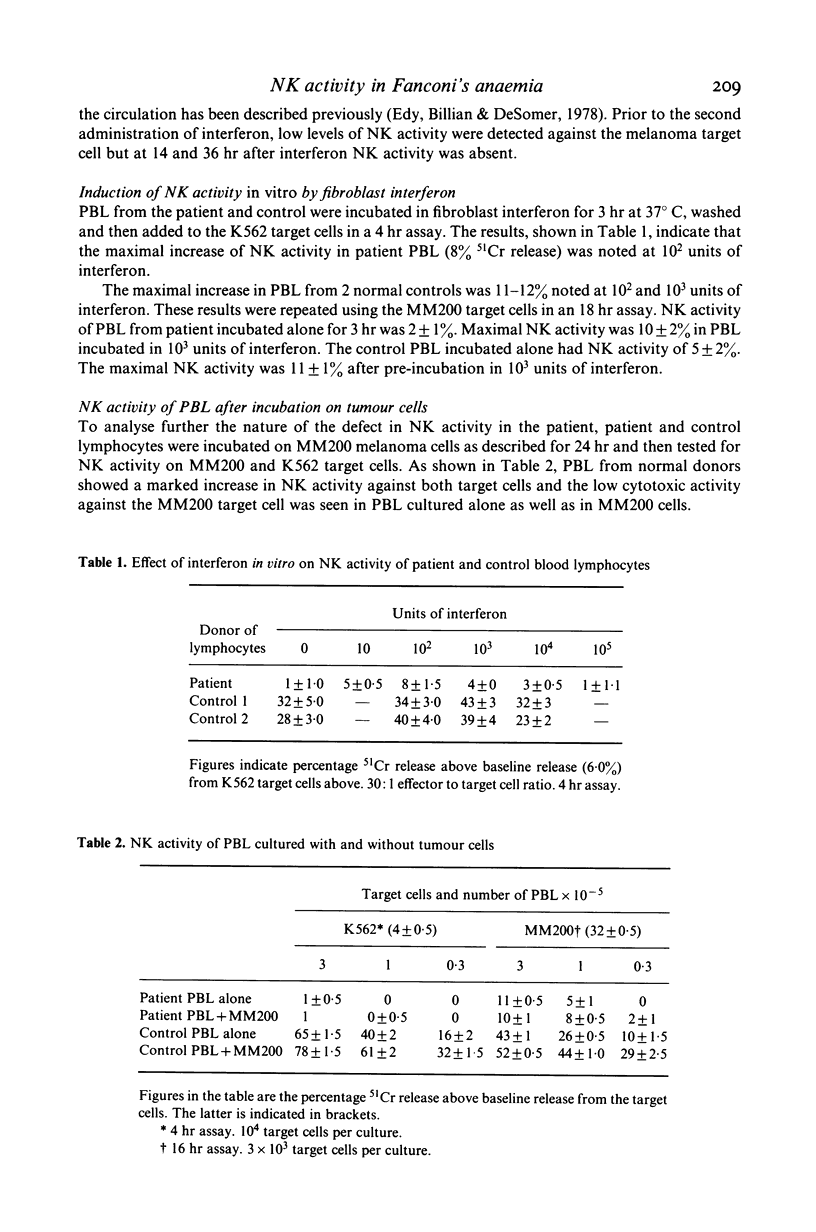
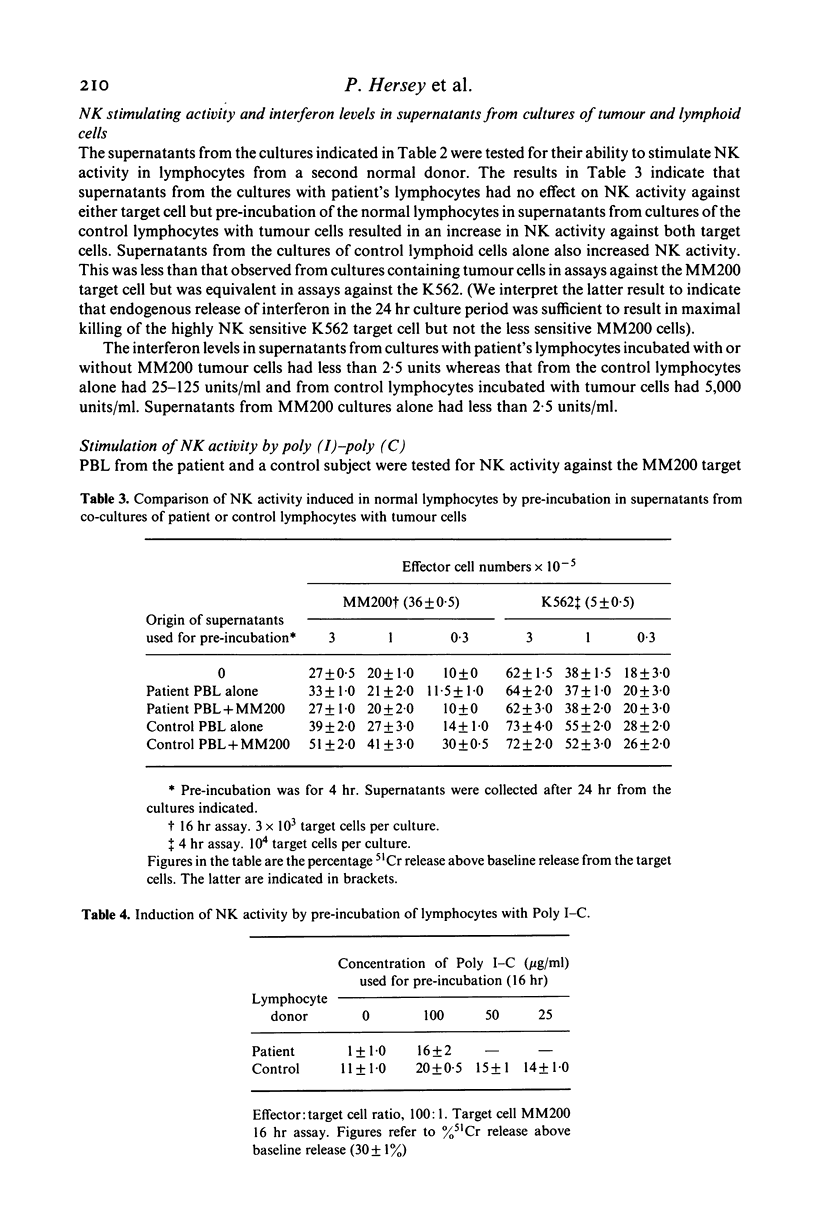
A child with Fanconi's anaemia diagnosed at 7 years of age presented in adult life with lymphopenia, recurrent warts and Bowen's disease. The latter resulted in the development of multiple cutaneous squamous cell carcinomas which metastasized to the skeleton. Investigation of her immune function revealed selective defects in natural killer (NK) cell activity. Humoral immunity and several tests of cell-mediated responses were within normal or became normal after treatment with levamisole or transfer factor. Analysis of the defect in NK activity revealed that low levels could be induced in vitro by fibroblast interferon. Stimulation of blood lymphocytes from the patient with the interferon inducer poly (I)-poly (C) resulted in an increase in NK activity but incubation of her lymphocytes on tumour cells did not result in an increase in NK activity or the release of interferon. This contrasted with the marked increase in NK activity and interferon release observed when lymphocytes from normal controls were incubated on tumor cells. These findings suggested the absence of NK activity in this patient was secondary to a defect in interferon release from lymphocytes on exposure to tumour antigens. It is considered that these defects may have been an important predisposing factor in the development of malignancy in this patient and possibly other patients with Fanconi's anaemia.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beard M. E., Young D. E., Bateman C. J., McCarthy G. T., Smith M. E., Sinclair L., Franklin A. W., Scott R. B. Fanconi's anaemia. Q J Med. 1973 Apr;42(166):403–422. [PubMed] [Google Scholar]
- Edy V. G., Billiau A., De Somer P. Non-appearance of injected fibroblast interferon in circulation. Lancet. 1978 Feb 25;1(8061):451–452. doi: 10.1016/s0140-6736(78)91251-5. [DOI] [PubMed] [Google Scholar]
- Grohmann P., Holmes W., Porzsolt F., Quirt I., Miller R. G., Phillips R. A. Stimulation of human NK-cell activity by cultured cells. I. Response of normals. Int J Cancer. 1978 Nov 15;22(5):528–534. doi: 10.1002/ijc.2910220504. [DOI] [PubMed] [Google Scholar]
- Hersey P., Edwards A., Edwards J. Characterization of mononuclear effector cells in human blood. Clin Exp Immunol. 1976 Jan;23(1):104–113. [PMC free article] [PubMed] [Google Scholar]
- Hersey P., Edwards A., McCarthy W. H. Tumour-related changes in natural killer cell activity in melanoma patients. Influence of stage of disease, tumour thickness and age of patients. Int J Cancer. 1980 Feb 15;25(2):187–194. doi: 10.1002/ijc.2910250204. [DOI] [PubMed] [Google Scholar]
- Hersey P., Wotherspoon J., Reid G., Gunz F. W. Hypogammaglobulinaemia associated with abnormalities of both B and T lymphocytes in patients with chronic lymphatic leukaemia. Clin Exp Immunol. 1980 Mar;39(3):698–707. [PMC free article] [PubMed] [Google Scholar]
- Hershey P., Edwards A., Milton G. W., McCarthy W. H. Relationship of cell-mediated cytotoxicity against melanoma cells to prognosis in melanoma patients. Br J Cancer. 1978 Apr;37(4):505–513. doi: 10.1038/bjc.1978.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling R., Haller O. Natural killer cells in the mouse: an alternative immune surveillance mechanism? Contemp Top Immunobiol. 1978;8:171–201. doi: 10.1007/978-1-4684-0922-2_6. [DOI] [PubMed] [Google Scholar]
- Lipton J. M., Nathan D. G. Aplastic and hypoplastic anemia. Pediatr Clin North Am. 1980 May;27(2):217–235. doi: 10.1016/s0031-3955(16)33848-2. [DOI] [PubMed] [Google Scholar]
- Pedersen F. K., Hertz H., Lundsteen C., Platz P., Thomsen M. Indication of primary immune deficiency in Fanconi's anemia. Acta Paediatr Scand. 1977 Nov;66(6):745–751. doi: 10.1111/j.1651-2227.1977.tb07983.x. [DOI] [PubMed] [Google Scholar]
- Roder J. C., Haliotis T., Klein M., Korec S., Jett J. R., Ortaldo J., Heberman R. B., Katz P., Fauci A. S. A new immunodeficiency disorder in humans involving NK cells. Nature. 1980 Apr 10;284(5756):553–555. doi: 10.1038/284553a0. [DOI] [PubMed] [Google Scholar]
- Santoli D., Koprowski H. Mechanisms of activation of human natural killer cells against tumor and virus-infected cells. Immunol Rev. 1979;44:125–163. doi: 10.1111/j.1600-065x.1979.tb00269.x. [DOI] [PubMed] [Google Scholar]
- Swift M., Zimmerman D., McDonough E. R. Squamous cell carcinomas in Fanconi's anemia. JAMA. 1971 Apr 12;216(2):325–326. [PubMed] [Google Scholar]
- Trinchieri G., Santoli D., Dee R. R., Knowles B. B. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Identification of the anti-viral activity as interferon and characterization of the human effector lymphocyte subpopulation. J Exp Med. 1978 May 1;147(5):1299–1313. doi: 10.1084/jem.147.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virelizier J. L., Griscelli C. Défaut sélectif de sécrétion d'interféron associé à un déficit d'activité cytotoxique naturelle. Arch Fr Pediatr. 1981 Feb;38(2):77–81. [PubMed] [Google Scholar]


