Abstract
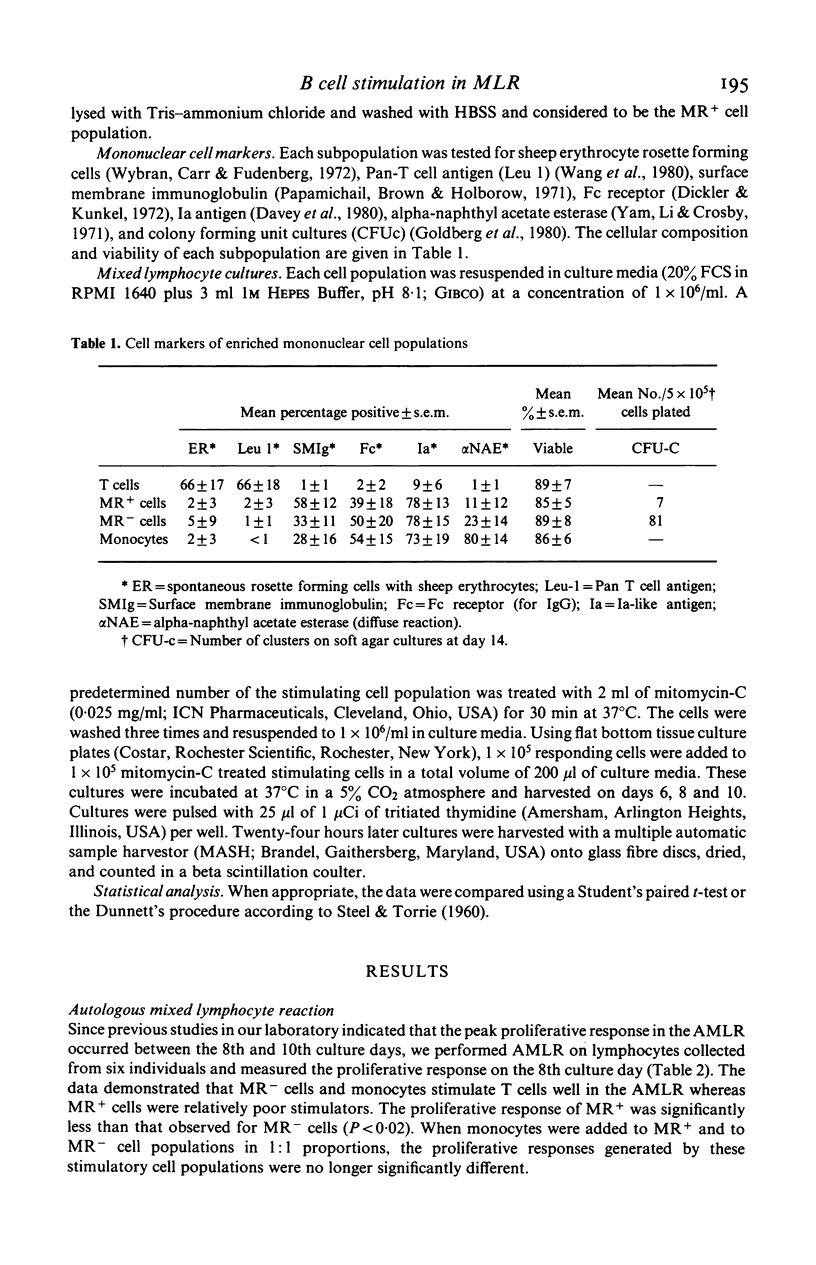
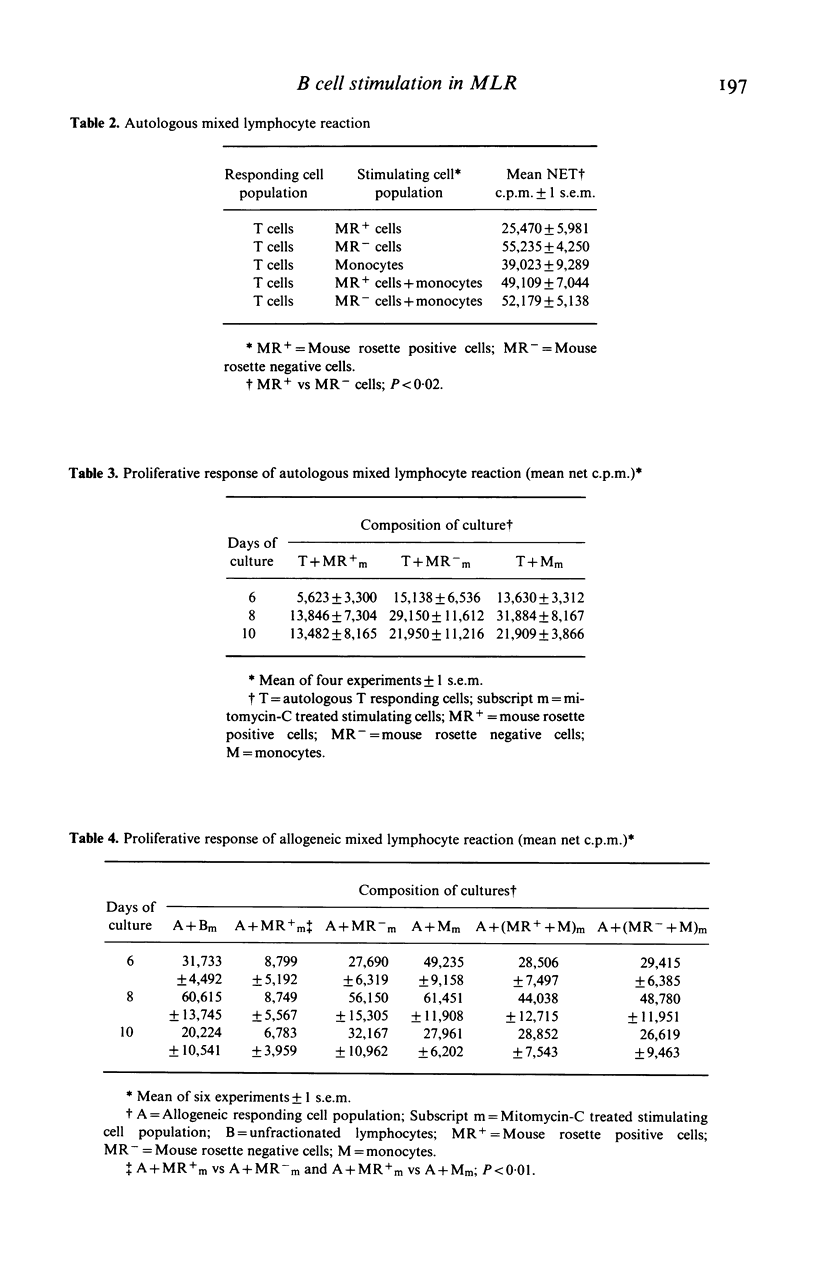
Mouse erythrocytes form spontaneous rosettes with a population of B lymphocytes from normal individuals and in the majority of lymphocytes from patients with B cell chronic lymphocytic leukaemia (CLL). We have compared the ability of mouse rosette positive (MR+) cells with mouse rosette negative (MR-) cells and monocytes to act as stimulators in the autologous mixed lymphocyte reaction (AMLR) and allogeneic mixed lymphocyte reaction (MLR). Mononuclear cells from the peripheral blood of healthy individuals were fractionated into T cells, MR+ cells, MR- cells and monocytes. Lymphocyte cultures were harvested on days 6, 8 and 10 and the incorporation of tritiated thymidine was determined. MR- cells and monocytes were potent stimulators in the AMLR and MLR. In contrast MR+ cells, like B cells from patients with CLL, stimulated less in the AMLR and MLR. We conclude that MR+ cells from normal individuals function similarly to cells from CLL in the AMLR and MLR.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale M. G., MacDermott R. P., Stacey M. C., Nash G. S., Hahn B. H., Seiden M. V., Jacobs S. L., Loewenstein L. S. Stimulating cell types in the autologous mixed leukocyte reaction in man. J Immunol. 1980 Jan;124(1):227–232. [PubMed] [Google Scholar]
- Bergholtz B., Albrechtsen D., Thorsby E. Stimulation of T lymphocytes by autologous non-T lymphoid cells. Participation of HLA-D? Tissue Antigens. 1977 Jul;10(1):27–38. doi: 10.1111/j.1399-0039.1977.tb00748.x. [DOI] [PubMed] [Google Scholar]
- Bertoglio J., Thierry C., Flores G., Boucharel C., Dore J. F. Mouse red cell rosette formation by subpopulations of human lymphocytes. Clin Exp Immunol. 1977 Jan;27(1):172–177. [PMC free article] [PubMed] [Google Scholar]
- Burns G. F., Cawley J. C. Spontaneous mouse erythrocyte-rosette formation: correlation with surface immunoglobulin phenotype in hairy-cell leukaemia. Clin Exp Immunol. 1980 Jan;39(1):83–89. [PMC free article] [PubMed] [Google Scholar]
- Catovsky D., Cherchi M., Okos A., Hegde U., Galton D. A. Mouse red-cell rosettes in B-lymphoproliferative disorders. Br J Haematol. 1976 Jun;33(2):173–177. doi: 10.1111/j.1365-2141.1976.tb03528.x. [DOI] [PubMed] [Google Scholar]
- Catovsky D., Papamichail M., Okos A., Miliani E., Holborow E. J. Formation of mouse red cell rosettes by "hairy" cells. Biomedicine. 1975 Apr 10;23(3):81–84. [PubMed] [Google Scholar]
- Catovsky D., Wechsler A., Cherchi M. Characterization of B-cell leukemias: a tentative immunomorphological scheme. Blood. 1981 Aug;58(2):410–411. [PubMed] [Google Scholar]
- Chapman E. H., Kurec A. S., Davey F. R. Cell volumes of normal and malignant mononuclear cells. J Clin Pathol. 1981 Oct;34(10):1083–1090. doi: 10.1136/jcp.34.10.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey F. R., Dock N. L., Wolos J. A. Studies of lymphocyte proliferation in hairy cell leukaemia: activity in mixed lymphocyte reaction and responses to mitogens. Br J Haematol. 1980 May;45(1):29–39. doi: 10.1111/j.1365-2141.1980.tb03808.x. [DOI] [PubMed] [Google Scholar]
- Dickler H. B., Kunkel H. G. Interaction of aggregated -globulin with B lymphocytes. J Exp Med. 1972 Jul 1;136(1):191–196. doi: 10.1084/jem.136.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dock N. L., Davey F. R. Peripheral blood monocytes in the autologous mixed lymphocyte reaction. Clin Immunol Immunopathol. 1980 Sep;17(1):123–131. doi: 10.1016/0090-1229(80)90080-x. [DOI] [PubMed] [Google Scholar]
- Dolen J. G., Park B. H. An improved micro-method for enumeration of human B-cell rosettes with mouse red blood cells. Immunol Commun. 1978;7(6):677–680. doi: 10.3109/08820137809068728. [DOI] [PubMed] [Google Scholar]
- Goldberg J., McGuire L. A., Dock N. L., Williams W. J., Davey F. R. Purification of human peripheral blood colony forming cells (CFUC). Exp Hematol. 1980 Oct;8(9):1086–1093. [PubMed] [Google Scholar]
- Gupta S., Good R. A., Siegal F. P. Rosette-formation with mouse erythrocytes. II. A marker for human B and non-T lymphocytes. Clin Exp Immunol. 1976 Aug;25(2):319–327. [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Grieco M. H. Rosette formation with mouse erythrocytes: probable marker for human B lymphocytes. Int Arch Allergy Appl Immunol. 1975;49(6):734–742. doi: 10.1159/000231457. [DOI] [PubMed] [Google Scholar]
- Gupta S., Pahwa R., O'Reilly R., Good R. A., Siegal F. P. Ontogeny of lymphocyte subpopulations in human fetal liver. Proc Natl Acad Sci U S A. 1976 Mar;73(3):919–922. doi: 10.1073/pnas.73.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman P. B., Raff H. V., Gilbert R. C., Picker L. J., Stobo J. D. T cells and macrophages involved in the autologous mixed lymphocyte reaction are required for the response to conventional antigen. J Immunol. 1980 Sep;125(3):1374–1379. [PubMed] [Google Scholar]
- Huber C., Fink U., Leibold W., Schmalzl F., Peterson P. A., Klareskog L., Braunsteiner H. The role of adherent HLA-DR+ mononuclear cells in autologous and allogeneic MLR. J Immunol. 1981 Aug;127(2):726–731. [PubMed] [Google Scholar]
- Koziner B., Filippa D. A., Mertelsmann R., Gupta S., Clarkson B., Good R. A., Siegal F. P. Characterization of malignant lymphomas in leukemic phase by multiple differentiation markers of mononuclear cells. Correlations with clinical features and conventional morphology. Am J Med. 1977 Oct;63(4):556–567. doi: 10.1016/0002-9343(77)90201-7. [DOI] [PubMed] [Google Scholar]
- Koziner B., Kempin S., Passe S., Gee T., Good R. A., Clarkson B. D. Characterization of B-cell leukemias: a tentative immunomorphological scheme. Blood. 1980 Nov;56(5):815–823. [PubMed] [Google Scholar]
- Kuntz M. M., Innes J. B., Weksler M. E. Lymphocyte transformation induced by autologous cells. IV. Human T-lymphocyte proliferation induced by autologous or allogeneic non-T lymphocytes. J Exp Med. 1976 May 1;143(5):1042–1054. doi: 10.1084/jem.143.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott R. P., Stacey M. C. Further characterization of the human autologous mixed leukocyte reaction (MLR). J Immunol. 1981 Feb;126(2):729–734. [PubMed] [Google Scholar]
- McGraw D. J., Kurec A. S., Davey F. R. Mouse erythrocyte formation. A marker for resting B lymphocytes. Am J Clin Pathol. 1982 Feb;77(2):177–183. doi: 10.1093/ajcp/77.2.177. [DOI] [PubMed] [Google Scholar]
- Mohanakumar T., Haar J. L., Russell E. C., Scott R. B., James G. W., 3rd, Kaplan A. M. Ultrastructural and immunologic studies of leukocytes which form rosettes with mouse erythrocytes in human lymphocytic leukemia. J Reticuloendothel Soc. 1979 Dec;26(6):803–813. [PubMed] [Google Scholar]
- Papamichail M., Brown J. C., Holborow E. J. Immunoglobulins on the surface of human lymphocytes. Lancet. 1971 Oct 16;2(7729):850–852. doi: 10.1016/s0140-6736(71)90224-8. [DOI] [PubMed] [Google Scholar]
- Stathopoulos G., Elliott E. V. Formation of mouse or sheep red-blood-cell rosettes by lymphocytes from normal and leukaemic individuals. Lancet. 1974 Apr 6;1(7858):600–601. doi: 10.1016/s0140-6736(74)92655-5. [DOI] [PubMed] [Google Scholar]
- Wang C. Y., Good R. A., Ammirati P., Dymbort G., Evans R. L. Identification of a p69,71 complex expressed on human T cells sharing determinants with B-type chronic lymphatic leukemic cells. J Exp Med. 1980 Jun 1;151(6):1539–1544. doi: 10.1084/jem.151.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolos J. A., Davey F. R. B lymphocyte function in B cell chronic lymphocytic leukaemia. Br J Haematol. 1981 Nov;49(3):395–403. doi: 10.1111/j.1365-2141.1981.tb07242.x. [DOI] [PubMed] [Google Scholar]
- Wolos J. A., Davey F. R. Depressed stimulation in the MLR by B lymphocytes on chronic lymphocytic leukemia: failure to demonstrate a suppressor cell. Clin Immunol Immunopathol. 1979 Sep;14(1):77–85. doi: 10.1016/0090-1229(79)90128-4. [DOI] [PubMed] [Google Scholar]
- Wolos J. A., Davey F. R. Function of lymphocyte subpopulations in chronic lymphocytic leukemia. Activity in the allogeneic and autologous mixed lymphocyte reaction. Cancer. 1980 Mar 1;45(5):893–898. doi: 10.1002/1097-0142(19800301)45:5<893::aid-cncr2820450511>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Wolos J. A., Davey F. R. T-lymphocyte function in B-cell chronic lymphocytic leukemia. Clin Immunol Immunopathol. 1980 Dec;17(4):573–583. doi: 10.1016/0090-1229(80)90153-1. [DOI] [PubMed] [Google Scholar]
- Wybran J., Carr M. C., Fudenberg H. H. The human rosette-forming cell as a marker of a population of thymus-derived cells. J Clin Invest. 1972 Oct;51(10):2537–2543. doi: 10.1172/JCI107069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]


