Abstract
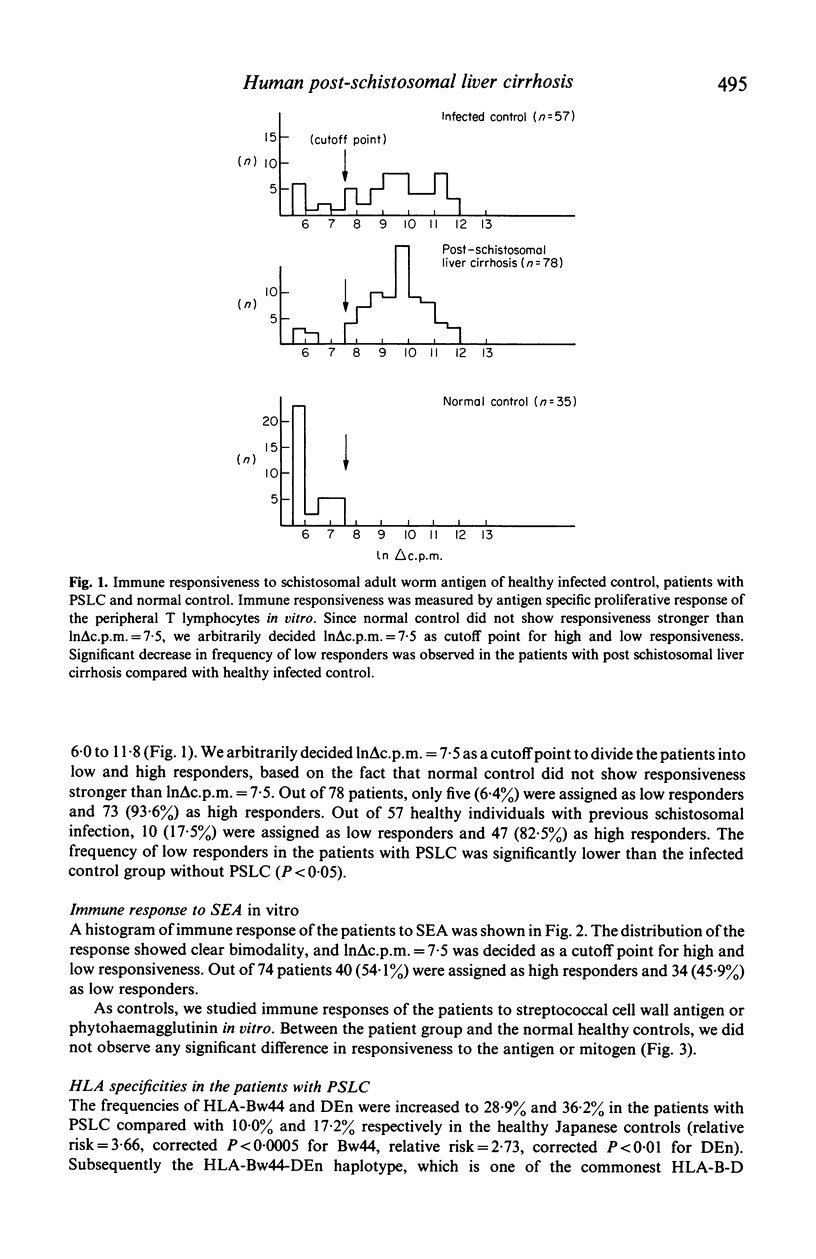
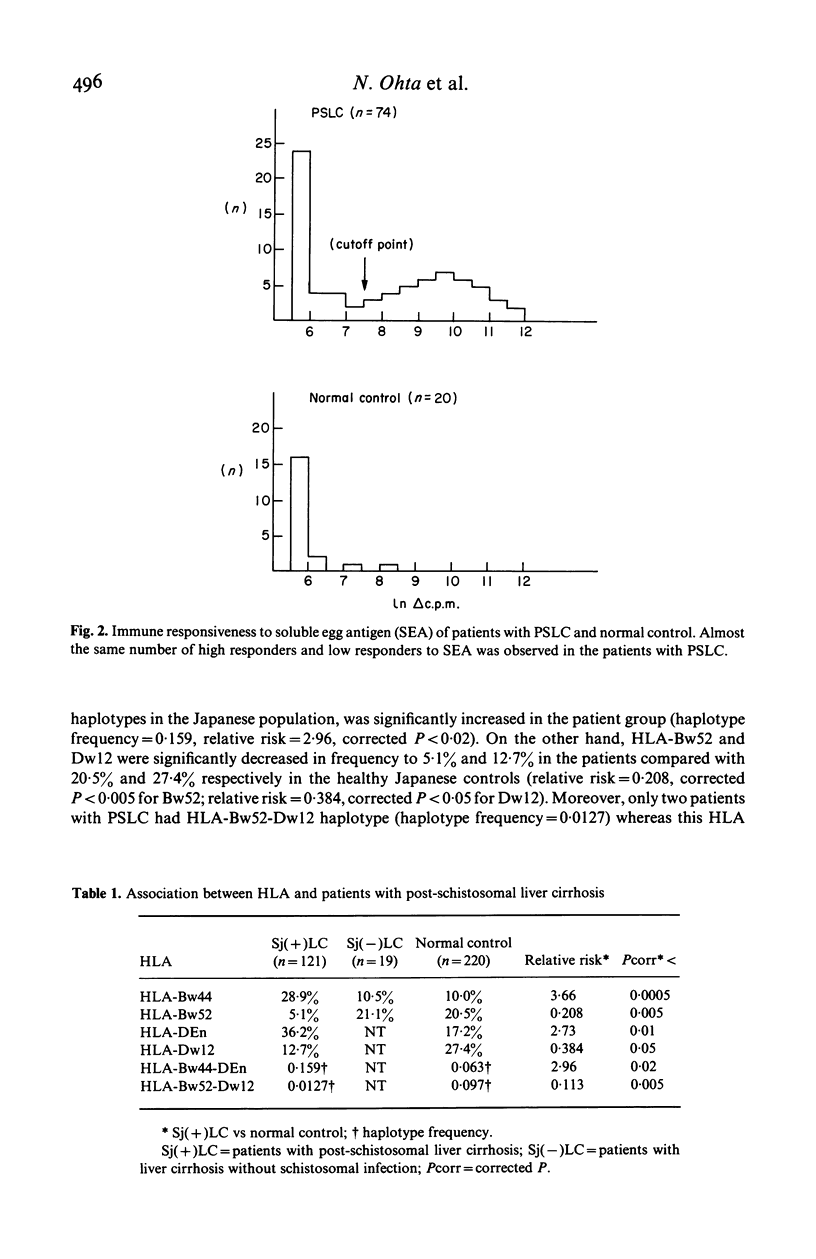
Immune responsiveness of 121 patients with post-schistosomal liver cirrhosis to schistosomal antigens was investigated. Out of 78 patients, only five (6.4%) showed low responsiveness to schistosomal adult worm antigen whereas 73 (93.6%) were high responders. Out of 57 healthy individuals with previous schistosomal infection, low responders were found in 17.5%. The frequency of low responders to schistosomal adult worm antigen was significantly decreased in the patients with post-schistosomal liver cirrhosis (P less than 0.05). Out of 121 patients, a significant increase in frequency of HLA-Bw44-DEn haplotype was observed (corrected P less than 0.02). On the other hand, HLA-Bw52-Dw12 haplotype which was reported to be in strong linkage disequilibrium with an immune suppression gene for schistosomal adult worm antigen was significantly decreased (corrected P less than 0.005). These observations suggested that an HLA-linked immune suppression gene controlled susceptibility or resistance to post-schistosomal liver cirrhosis through regulation of immune responsiveness of the hosts to schistosomal antigen in man.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benacerraf B., McDevitt H. O. Histocompatibility-linked immune response genes. Science. 1972 Jan 21;175(4019):273–279. doi: 10.1126/science.175.4019.273. [DOI] [PubMed] [Google Scholar]
- Boros D. L., Warren K. S. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970 Sep 1;132(3):488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan R. D., Fine D. P., Colley D. G. Schistosoma mansoni infection in mice depleted of thymus-dependent lymphocytes. II. Pathology and altered pathogenesis. Am J Pathol. 1973 May;71(2):207–218. [PMC free article] [PubMed] [Google Scholar]
- CHAFFEE E. F., BAUMAN P. M., SHAPILO J. J. Diagnosis of schistosomiasis by complement-fixation. Am J Trop Med Hyg. 1954 Sep;3(5):905–913. doi: 10.4269/ajtmh.1954.3.905. [DOI] [PubMed] [Google Scholar]
- Colley D. G. Adoptive suppression of granuloma formation. J Exp Med. 1976 Mar 1;143(3):696–700. doi: 10.1084/jem.143.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley D. G., Cook J. A., Freeman G. L., Jr, Bartholomew R. K., Jordan P. Immune responses during human schistosomiasis mansoni. I. In vitro lymphocyte blastogenic responses to heterogeneous antigenic preparations from schistosome eggs, worms and cercariae. Int Arch Allergy Appl Immunol. 1977;53(5):420–433. [PubMed] [Google Scholar]
- Colley D. G., Hieny S. E., Bartholomew R. K., Cook J. A. Immune responses during human schistosomiasis mansoni. III. Regulatory effect of patient sera on human lymphocyte blastogenic responses to schistosome antigen preparations. Am J Trop Med Hyg. 1977 Sep;26(5 Pt 1):917–925. [PubMed] [Google Scholar]
- Doughty B. L., Phillips S. M. Delayed hypersensitivity granuloma formation around Schistosoma mansoni eggs in vitro. I. Definition of the model. J Immunol. 1982 Jan;128(1):30–36. [PubMed] [Google Scholar]
- Ellner J. J., Olds G. R., Kamel R., Osman G. S., el-Kholy A., Mahmoud A. A. Suppression splenic T lymphocytes in human hepatosplenic Schistosomiasis mansoni. J Immunol. 1980 Jul;125(1):308–312. [PubMed] [Google Scholar]
- Ellner J. J., Olds G. R., Osman G. S., El Kholy A., Mahmoud A. A. Dichotomies in the reactivity to worm antigen in human schistosomiasis mansoni. J Immunol. 1981 Jan;126(1):309–312. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nose Y., Komori K., Inouye H., Nomura K., Yamamura M., Tsuji K. Relationship between HLA-D and in vitro and in vivo responsiveness to Candida allergen. Clin Exp Immunol. 1980 May;40(2):345–350. [PMC free article] [PubMed] [Google Scholar]
- Ottesen E. A., Hiatt R. A., Cheever A. W., Sotomayor Z. R., Neva F. A. The acquisition and loss of antigen-specific cellular immune responsiveness in acute and chronic schistosomiasis in man. Clin Exp Immunol. 1978 Jul;33(1):37–47. [PMC free article] [PubMed] [Google Scholar]
- Pelley R. P., Warren K. S., Jordan P. Purified antigen radioimmunoassay in serological diagnosis of schistosomiasis mansoni. Lancet. 1977 Oct 15;2(8042):781–785. doi: 10.1016/s0140-6736(77)90722-x. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E., Tracy J. W., El Kholy A. Activation of antigen-specific suppressor cells in human schistosomiasis mansoni by fractions of soluble egg antigens nonadherent to Con A sepharose. J Immunol. 1981 Dec;127(6):2314–2318. [PubMed] [Google Scholar]
- Salam E. A., Ishaac S., Mahmoud A. A. Histocompatibilty-linked susceptibility for hepatospleenomegaly in human schistosomiasis mansoni. J Immunol. 1979 Oct;123(4):1829–1831. [PubMed] [Google Scholar]
- Sasazuki T., Kaneoka H., Nishimura Y., Kaneoka R., Hayama M., Ohkuni H. An HLA-linked immune suppression gene in man. J Exp Med. 1980 Aug 1;152(2 Pt 2):297s–313s. [PubMed] [Google Scholar]
- Sasazuki T., Kohno Y., Iwamoto I., Tanimura M., Naito S. Association between an HLA haplotype and low responsiveness to tetanus toxoid in man. Nature. 1978 Mar 23;272(5651):359–361. doi: 10.1038/272359b0. [DOI] [PubMed] [Google Scholar]
- Sasazuki T., Ohta N., Kaneoka R., Kojima S. Association between an HLA haplotype and low responsiveness to schistosomal worm antigen in man. J Exp Med. 1980 Aug 1;152(2 Pt 2):314s–318s. [PubMed] [Google Scholar]
- Spencer M. J., Cherry J. D., Terasaki P. I. HL-A antigens and antibody response after influenza A vaccination. Decreased response associated with HL-A type W16. N Engl J Med. 1976 Jan 1;294(1):13–16. doi: 10.1056/NEJM197601012940104. [DOI] [PubMed] [Google Scholar]
- Warren K. S. Hepatosplenic schistosomiasis mansoni: an immunologic disease. Bull N Y Acad Med. 1975 Apr;51(4):545–550. [PMC free article] [PubMed] [Google Scholar]
- Warren K. S., Kellermeyer R. W., Jordan P., Littell A. S., Cook J. A., Kagan I. G. Immunologic diagnosis of schistosomiasis. I. A controlled study of intradermal (immediate and delayed) and serologic tests in St. Lucians infected with Schistosoma mansoni and in uninfected St. Vincentians. Am J Trop Med Hyg. 1973 Mar;22(2):189–198. doi: 10.4269/ajtmh.1973.22.189. [DOI] [PubMed] [Google Scholar]
- Warren K. S. The immunopathogenesis of schistosomiasis: a multidisciplinary approach. Trans R Soc Trop Med Hyg. 1972;66(3):417–434. doi: 10.1016/0035-9203(72)90273-8. [DOI] [PubMed] [Google Scholar]
- de Vries R. P., Kreeftenberg H. G., Loggen H. G., van Rood J. J. In vitro immune responsiveness to vaccinea virus and HLA. N Engl J Med. 1977 Sep 29;297(13):692–696. doi: 10.1056/NEJM197709292971303. [DOI] [PubMed] [Google Scholar]


