Abstract
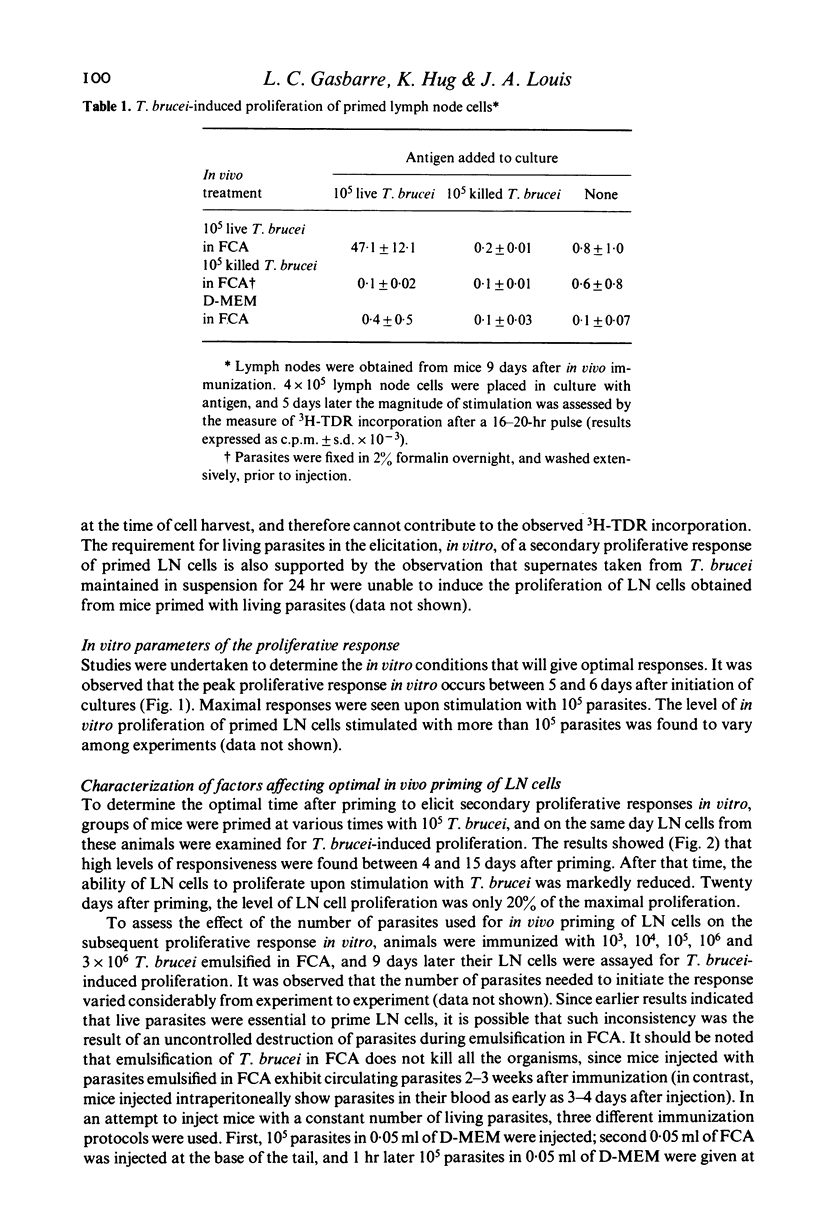
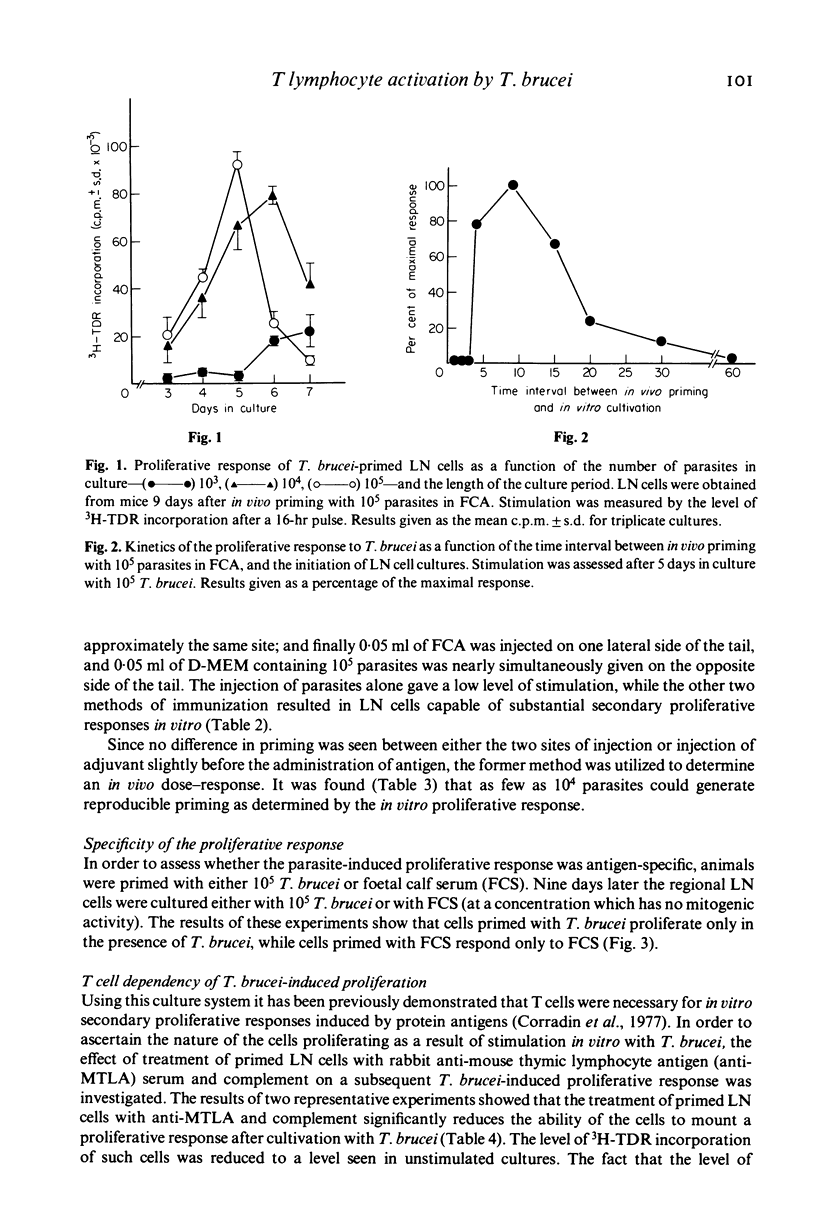
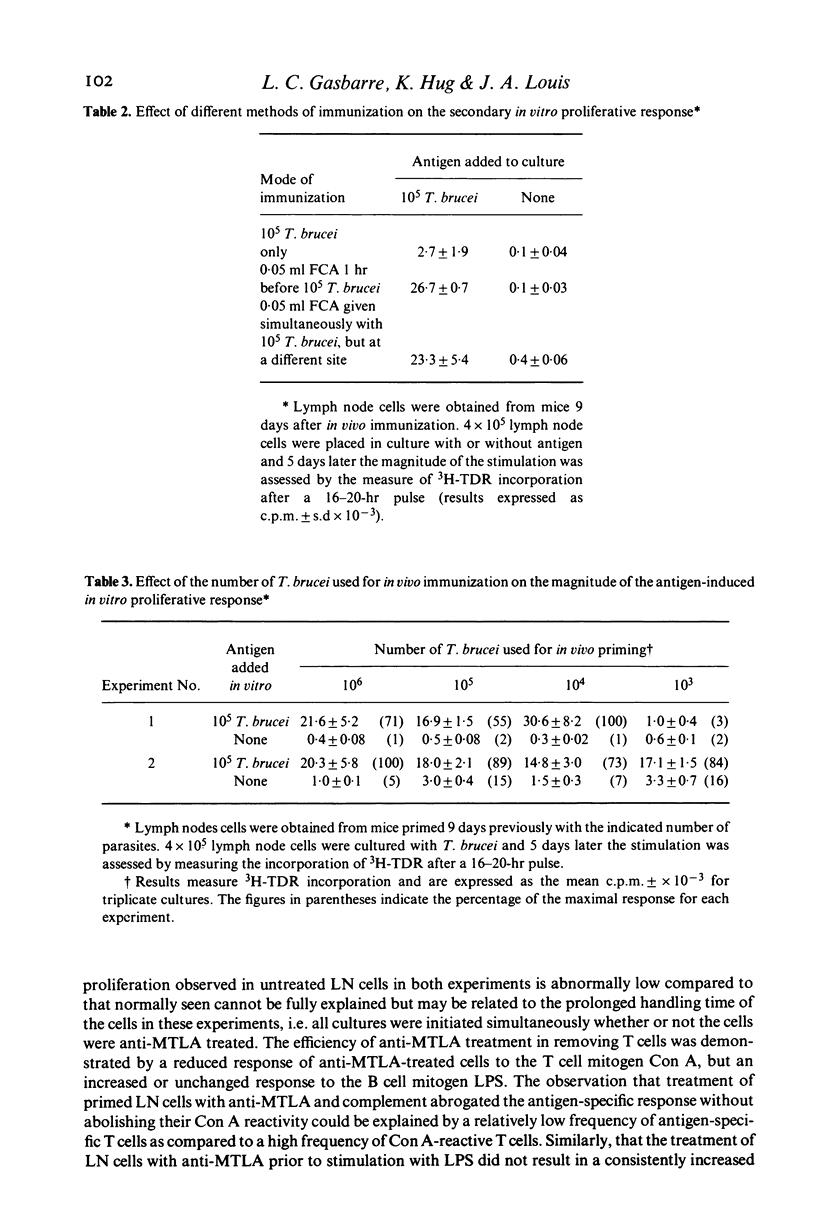
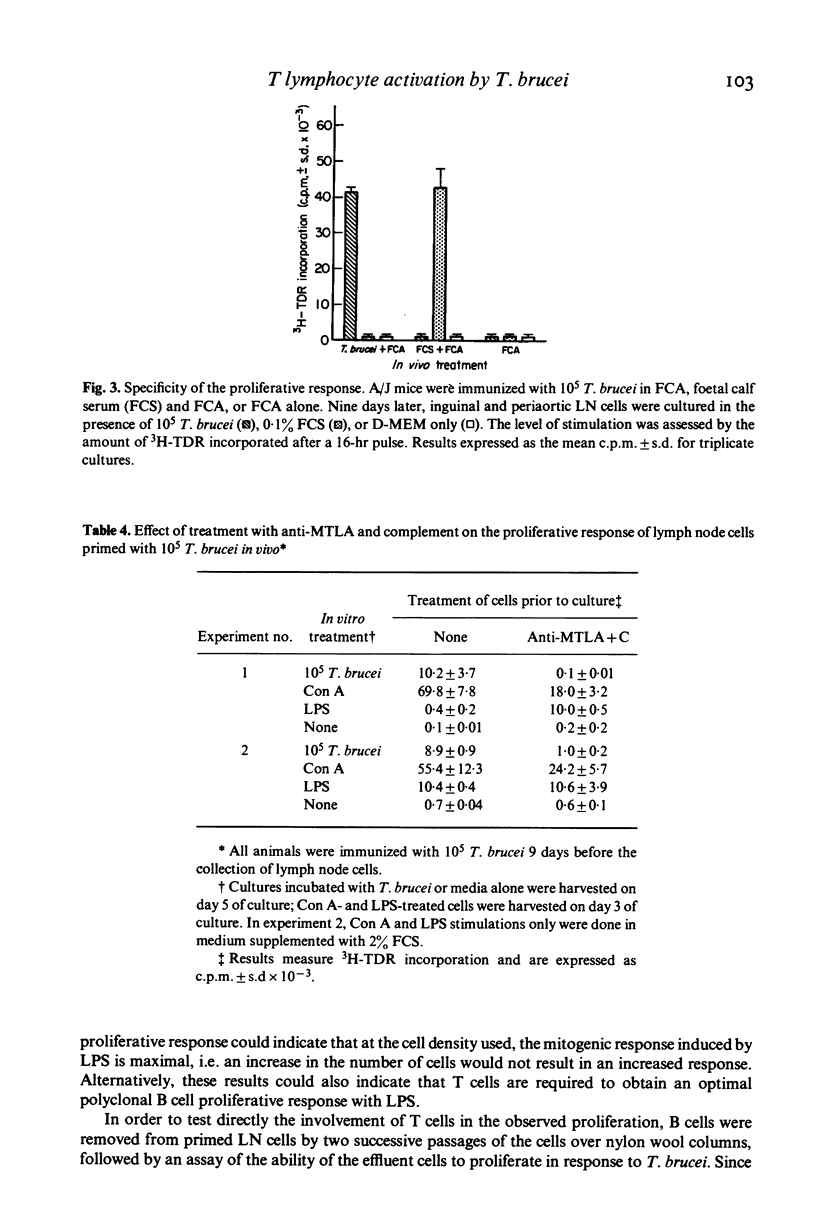
A procedure which results in the specific activation of primed murine T lymphocytes was adapted for the study of T lymphocyte activation by the African trypanosome: Trypanosoma brucei. The assay calls for the in vivo priming of lymphocytes by the subcutaneous administration of parasites, followed by the co-cultivation in vitro of cells taken from the regional draining lymph nodes and the parasite. This co-cultivation results in a marked proliferation of lymphoid cells. The proliferation was shown to be specific for the parasite, and to be dependent on the presence of T lymphocytes and macrophages. Both the in vivo priming and the in vitro activation were shown to require the presence of living parasites. Various factors influencing the magnitude of the proliferative response were analysed. Of special interest is the observation that the time interval between in vivo priming and in vitro culture which results in a substantial proliferative response is quite short when compared to that seen with other antigens. Although lymph node cells from mice primed with T. brucei 1 to 2 weeks previously are able to mount a secondary proliferative response upon stimulation with T. brucei, cells taken 3 weeks after priming are unresponsive to an in vitro challenge with T. brucei. This unresponsiveness may be a result of the generalized immunosuppression seen in African trypanosomiasis. Thus, this method offers the potential for the study of specific T cell responsiveness in African trypanosome infections.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Möller G., Sjöberg O. B lymphocytes can be stimulated by concanavalin A in the presence of humoral factors released by T cells. Eur J Immunol. 1972 Feb;2(1):99–101. doi: 10.1002/eji.1830020119. [DOI] [PubMed] [Google Scholar]
- Askonas B. A., Corsini A. C., Clayton C. E., Ogilvie B. M. Functional depletion of T- and B-memory cells and other lymphoid cell subpopulations-during trypanosomiasis. Immunology. 1979 Feb;36(2):313–321. [PMC free article] [PubMed] [Google Scholar]
- Campbell G. H., Esser K. M., Weinbaum F. I. Trypanosoma rhodesiense infection in B-cell-deficient mice. Infect Immun. 1977 Nov;18(2):434–438. doi: 10.1128/iai.18.2.434-438.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradin G., Etlinger H. M., Chiller J. M. Lymphocyte specificity to protein antigens. I. Characterization of the antigen-induced in vitro T cell-dependent proliferative response with lymph node cells from primed mice. J Immunol. 1977 Sep;119(3):1048–1053. [PubMed] [Google Scholar]
- Corsini A. C., Clayton C., Askonas B. A., Ogilvie B. M. Suppressor cells and loss of B-cell potential in mice infected with Trypanosoma brucei. Clin Exp Immunol. 1977 Jul;29(1):122–131. [PMC free article] [PubMed] [Google Scholar]
- Eardley D. D., Jayawardena A. N. Suppressor cells in mice infected with Trypanosoma brucei. J Immunol. 1977 Sep;119(3):1029–1033. [PubMed] [Google Scholar]
- Finerty J. F., Krehl E. P., McKelvin R. L. Delayed-type hypersensitivity in mice immunized with Trypanosoma rhodesiense antigens. Infect Immun. 1978 May;20(2):464–467. doi: 10.1128/iai.20.2.464-467.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houba V., Brown K. N., Allison A. C. Heterophile antibodies, M-antiglobulins and immunoglobulins in experimental trypanosomiasis. Clin Exp Immunol. 1969 Jan;4(1):113–123. [PMC free article] [PubMed] [Google Scholar]
- Hudson K. M., Byner C., Freeman J., Terry R. J. Immunodepression, high IgM levels and evasion of the immune response in murine trypanosomiasis. Nature. 1976 Nov 18;264(5583):256–258. doi: 10.1038/264256a0. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Louis J., Moedder E., Behin R., Engers H. Recognition of protozoan parasite antigens by murine T lymphocytes. I. Induction of specific T lymphocyte-dependent proliferative response to Leishmania tropica. Eur J Immunol. 1979 Nov;9(11):841–847. doi: 10.1002/eji.1830091103. [DOI] [PubMed] [Google Scholar]
- Mansfield J. M., Craig S. A., Stelzer G. T. Lymphocyte function in experimental african trypanosomiasis: mitogenic effects of trypanosome extracts in vitro. Infect Immun. 1976 Oct;14(4):976–981. doi: 10.1128/iai.14.4.976-981.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield J. M., Wallace J. H. Suppression of cell-mediated immunity in experimental African trypanosomiasis. Infect Immun. 1974 Aug;10(2):335–339. doi: 10.1128/iai.10.2.335-339.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. K., Jennings F. W., Murray M., Urquhart G. M. The nature of immunosuppression in Trypanosoma brucei infections in mice. II. The role of the T and B lymphocytes. Immunology. 1974 Nov;27(5):825–840. [PMC free article] [PubMed] [Google Scholar]
- Pearson T. W., Roelants G. E., Lundin L. B., Mayor-Withey K. S. Immune depression in trypanosome-infected mice. I. Depressed T lymphocyte responses. Eur J Immunol. 1978 Oct;8(10):723–727. doi: 10.1002/eji.1830081010. [DOI] [PubMed] [Google Scholar]
- Peck A. B., Click R. E. Immune responses in vitro. 3. Enhancement of the mouse mixed lymphocyte interaction by isologous and homologous sera. Eur J Immunol. 1973 Jul;3(7):385–392. doi: 10.1002/eji.1830030703. [DOI] [PubMed] [Google Scholar]
- Rosenwasser L. J., Rosenthal A. S. Adherent cell function in murine T lymphocyte antigen recognition. I. A. macrophage-dependent T cell proliferation assay in the mouse. J Immunol. 1978 Jun;120(6):1991–1995. [PubMed] [Google Scholar]
- Rosenwasser L. J., Rosenthal A. S. Adherent cell function in murine T lymphocyte antigen recognition. II. Definition of genetically restricted and nonrestricted macrophage functions in T cell proliferation. J Immunol. 1978 Dec;121(6):2497–2501. [PubMed] [Google Scholar]
- Tizard I. R., Soltys M. A. Cell-mediated hypersensitivity in rabbits infected with Trypanosoma brucei and Trypanosoma rhodesiense. Infect Immun. 1971 Dec;4(6):674–677. doi: 10.1128/iai.4.6.674-677.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B. Quantitative studies on the mixed lymphocyte interaction in rats. I. Conditions and parameters of response. J Exp Med. 1967 Oct 1;126(4):625–654. doi: 10.1084/jem.126.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]


