Abstract
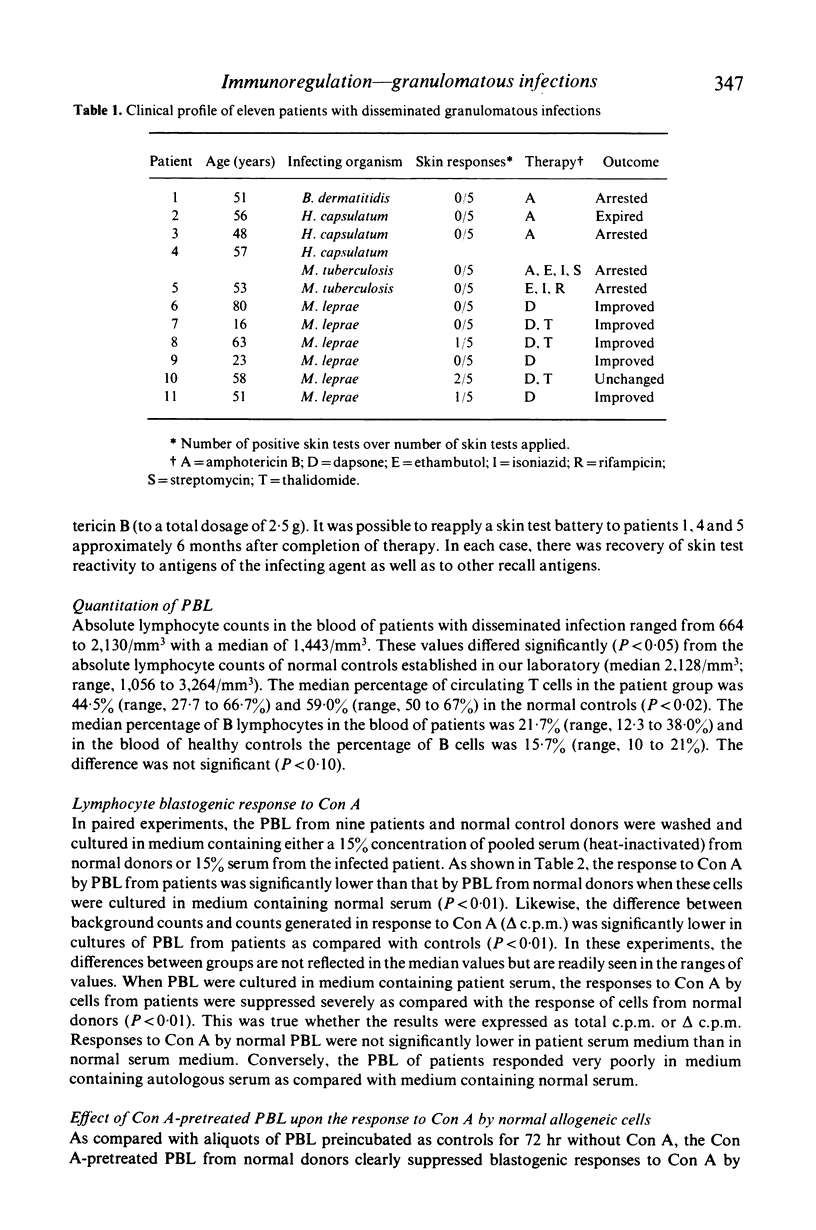
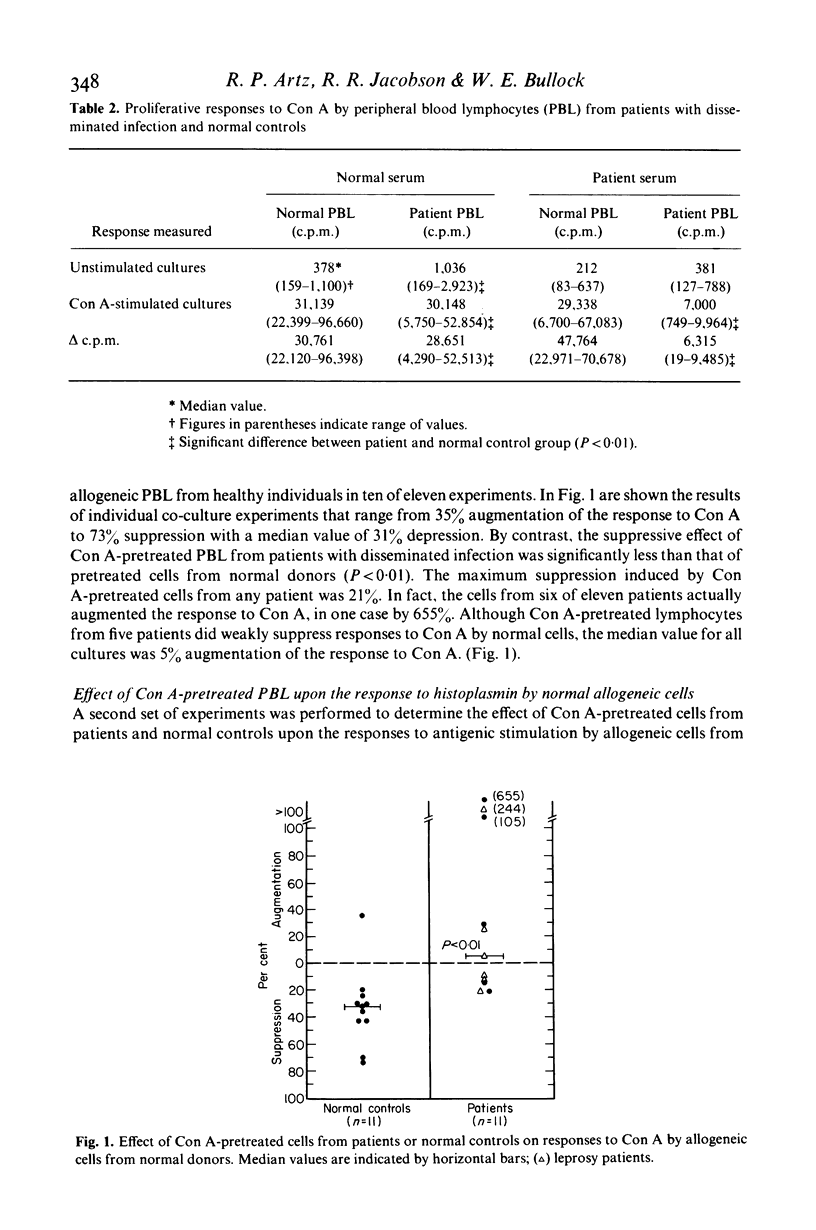
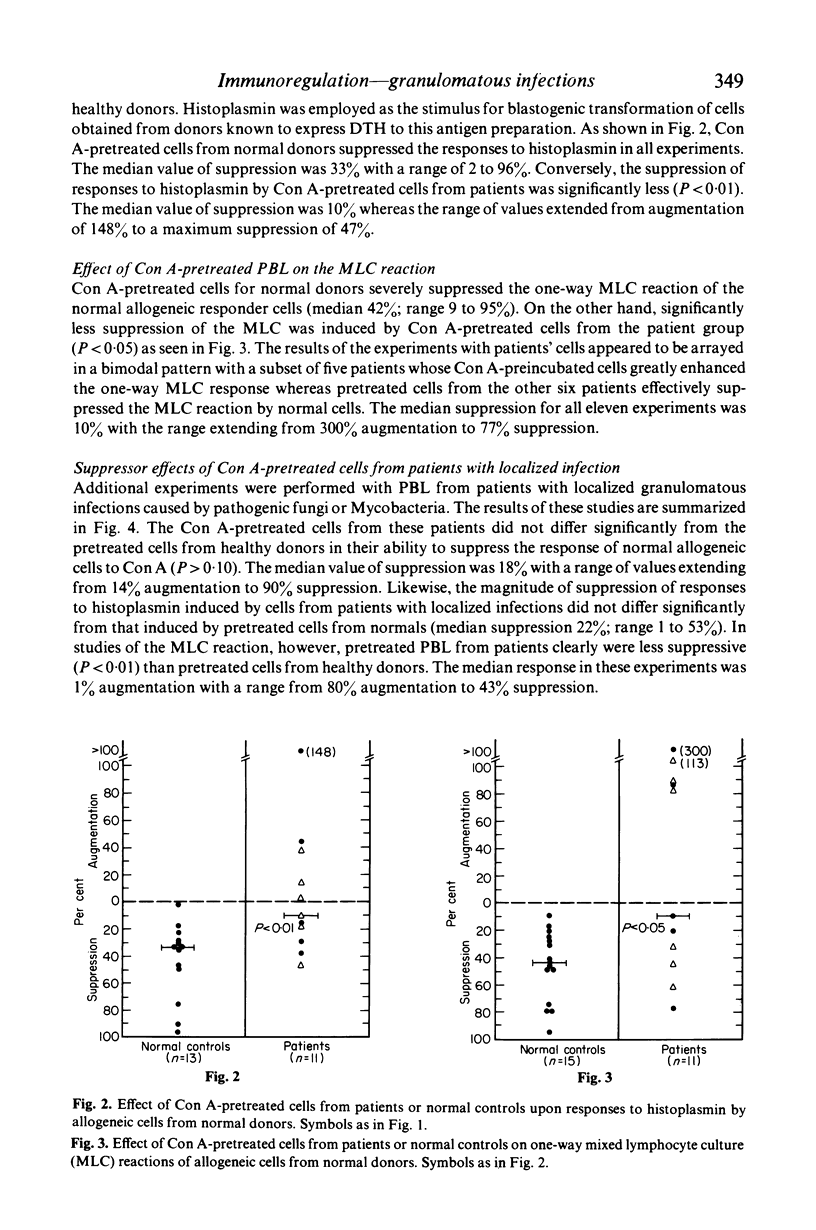
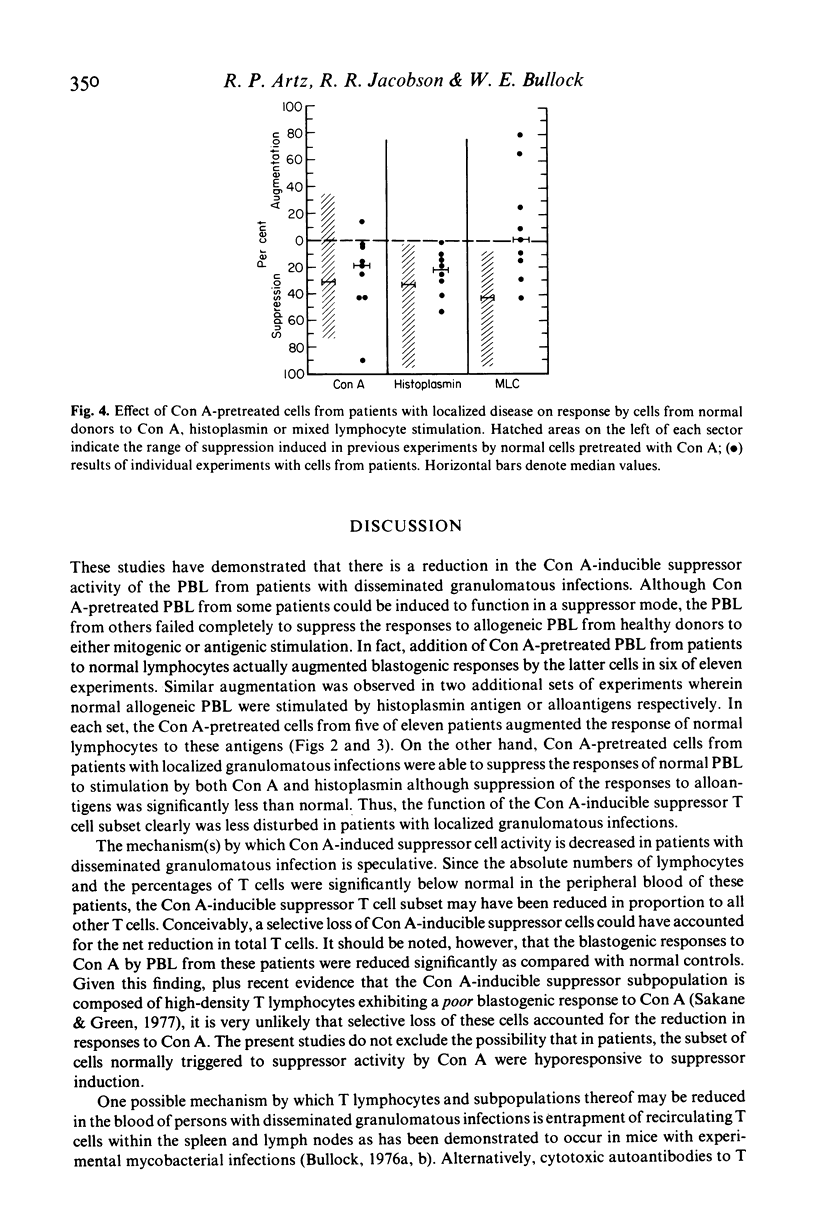
The effect of granulomatous infections upon the activity of a T lymphocyte subclass in human peripheral blood that can be induced by concanavalin A (Con A) to function in a suppressor mode was studied. Peripheral blood lymphocytes (PBL) from eleven patients with disseminated mycotic or mycobacterial infections or from controls were preincubated with and without Con A, washed and cultured with allogeneic PBL freshly drawn from healthy donors sensitive to histoplasmin. DNA synthesis was then measured in co-cultures stimulated by Con A, histoplasmin, or by the mixed lymphocyte culture (MLC) reaction alone. As compared with cells preincubated without Con A, the Con A-pretreated cells were significantly less effective in suppressing the responses of normal PBL to histoplasmin (P < 0.01), and in a one-way MLC reaction (P < 0.05). The Con A-induced suppressor activity of PBL from nine patients with localized granulomatous infections did not differ significantly from that exerted by PBL of normal controls in two of the three co-culture systems employed. These studies suggest that either dysfunction or a reduction of the Con A-inducible T-suppressor cell subpopulation in peripheral blood is frequent among patients was disseminated granulomatous infections.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Sagawa A., Pascual E., Hebert J., Sadeghee S. Suppressor T-cell abnormality in idiopathic systemic lupus erythematosus. Clin Immunol Immunopathol. 1976 Sep;6(2):192–199. doi: 10.1016/0090-1229(76)90110-0. [DOI] [PubMed] [Google Scholar]
- Bjune G. In vitro lymphocyte stimulation in leprosy; simultaneous stimulation with Mycobacterium leprae antigens and phytohaemagglutinin. Clin Exp Immunol. 1979 Jun;36(3):479–487. [PMC free article] [PubMed] [Google Scholar]
- Bresnihan B., Jasin H. E. Suppressor function of peripheral blood mononuclear cells in normal individuals and in patients with systemic lupus erythematosus. J Clin Invest. 1977 Jan;59(1):106–116. doi: 10.1172/JCI108607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock W. E., Jr Perturbation of lymphocyte circulation in experimental murine leprosy. I. Description of the defect. J Immunol. 1976 Oct;117(4):1164–1170. [PubMed] [Google Scholar]
- Bullock W. E., Jr Perturbation of lymphocyte circulation in experimental murine leprosy. II. Nature of the defect. J Immunol. 1976 Oct;117(4):1171–1178. [PubMed] [Google Scholar]
- Hodgson H. J., Wands J. R., Isselbacher K. J. Alteration in suppressor cell activity in chronic active hepatitis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1549–1553. doi: 10.1073/pnas.75.3.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson H. J., Wands J. R., Isselbacher K. J. Decreased suppressor cell activity in inflammatory bowel disease. Clin Exp Immunol. 1978 Jun;32(3):451–458. [PMC free article] [PubMed] [Google Scholar]
- Hubert C., Delespesse G., Govaerts A. Concanavalin A-activated suppressor cells in normal human peripheral blood lymphocytes. Clin Exp Immunol. 1976 Oct;26(1):95–98. [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisler J. M., Arnaiz A., Perez B., Bootello A. Lumphocytotoxins in leprosy. Int J Lepr Other Mycobact Dis. 1975 Apr-Jun;43(2):91–94. [PubMed] [Google Scholar]
- Mehra V., Mason L. H., Fields J. P., Bloom B. R. Lepromin-induced suppressor cells in patients with leprosy. J Immunol. 1979 Oct;123(4):1813–1817. [PubMed] [Google Scholar]
- Pernis B., Forni L., Amante L. Immunoglobulin spots on the surface of rabbit lymphocytes. J Exp Med. 1970 Nov;132(5):1001–1018. doi: 10.1084/jem.132.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff H. V., Cochrum K. C., Stobo J. D. Macrophage-T cell interactions in the Con A induction of human suppressive T cells. J Immunol. 1978 Dec;121(6):2311–2315. [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Sakane T., Green I. Human suppressor T cells induced by concanavalin A: suppressor T cells belong to distinctive T cell subclasses. J Immunol. 1977 Sep;119(3):1169–1178. [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Reeves J. P., Green I. Studies of immune functions of patients with systemic lupus erythematosus. Complement-dependent immunoglobulin M anti-thymus-derived cell antibodies preferentially inactivate suppressor cells. J Clin Invest. 1979 May;63(5):954–965. doi: 10.1172/JCI109396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou L., Schwartz S. A., Good R. A. Suppressor cell activity after concanavalin A treatment of lymphocytes from normal donors. J Exp Med. 1976 May 1;143(5):1100–1110. doi: 10.1084/jem.143.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D., Paul S., Van Scoy R. E., Hermans P. E. Suppressor thymus-derived lymphocytes in fungal infection. J Clin Invest. 1976 Feb;57(2):319–328. doi: 10.1172/JCI108283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelkauskas A. J., Callery R. T., McDowell J., Borel Y., Schlossman S. F. Direct evidence for loss of human suppressor cells during active autoimmune disease. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5150–5154. doi: 10.1073/pnas.75.10.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Durm M., Broder S., Blackman M., Blaese R. M., Strober W. Role of suppressor T cells in pathogenesis of common variable hypogammaglobulinaemia. Lancet. 1974 Sep 14;2(7881):609–613. doi: 10.1016/s0140-6736(74)91940-0. [DOI] [PubMed] [Google Scholar]


