Abstract
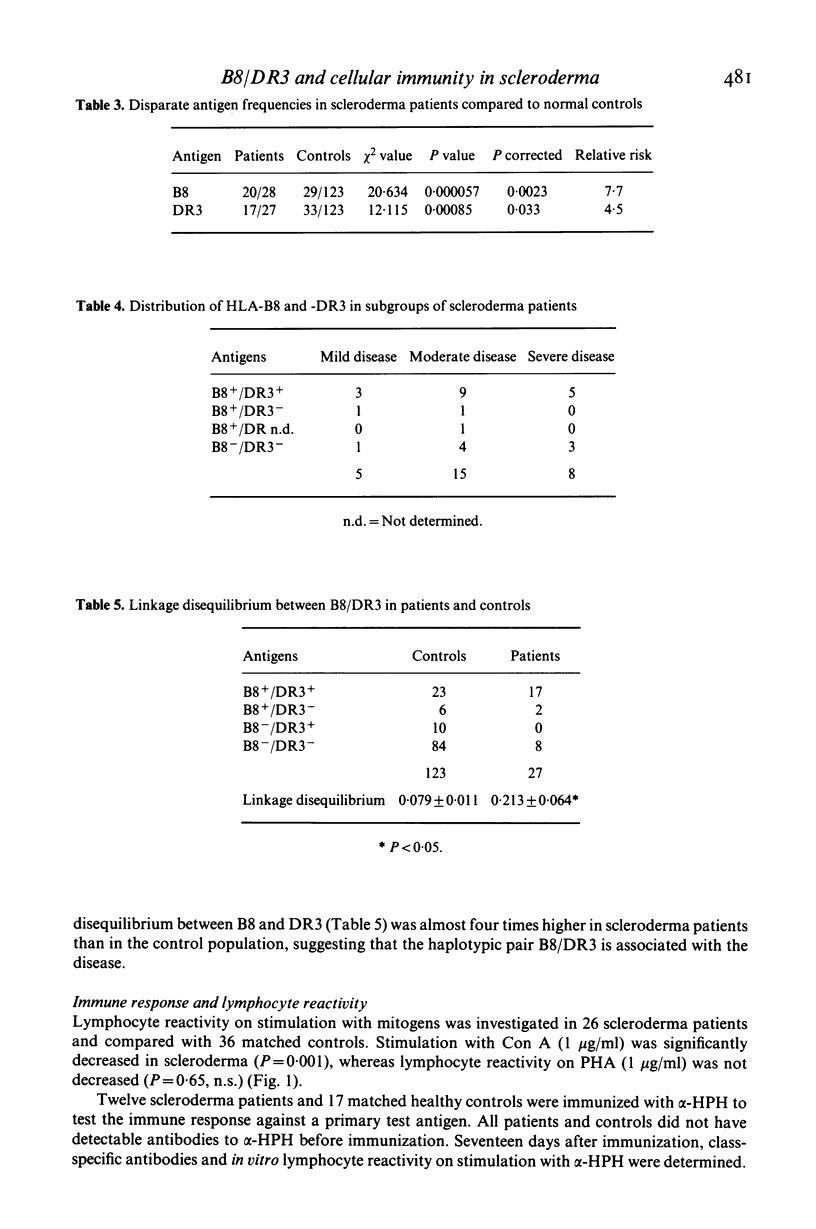
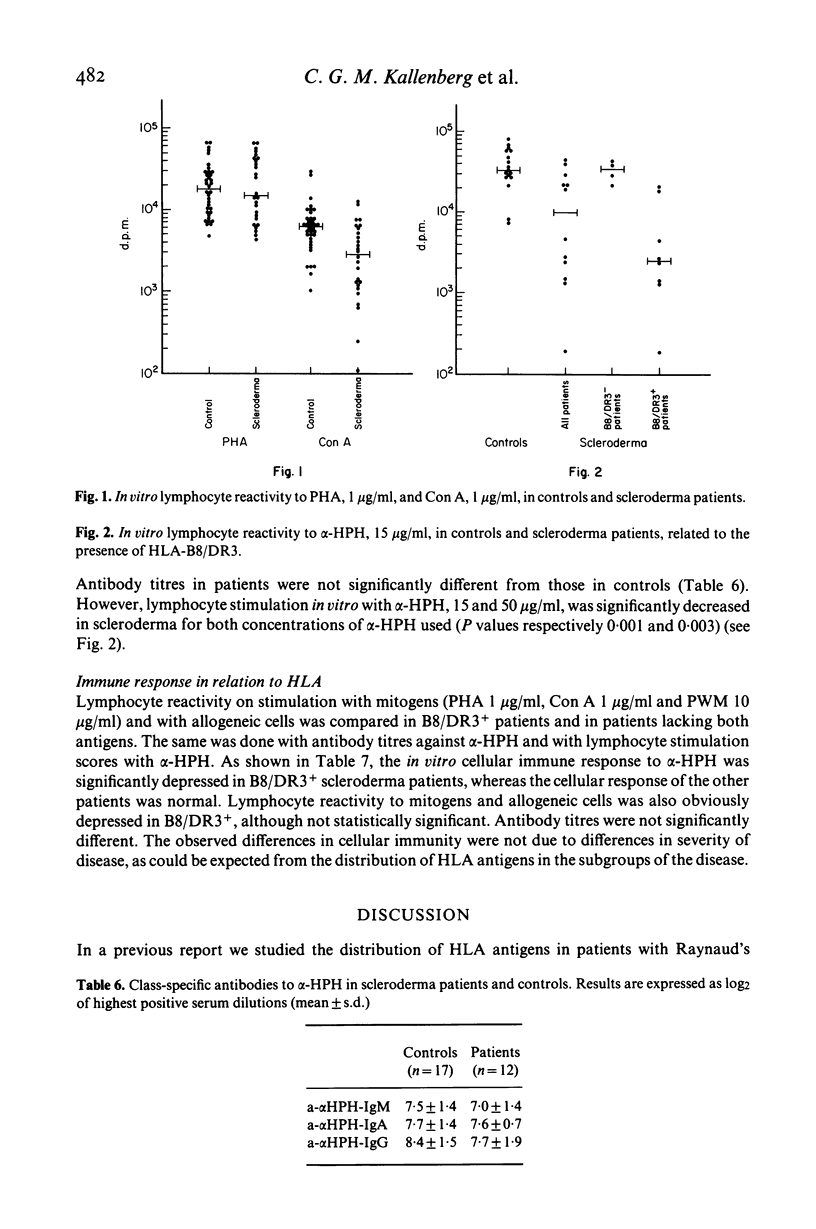
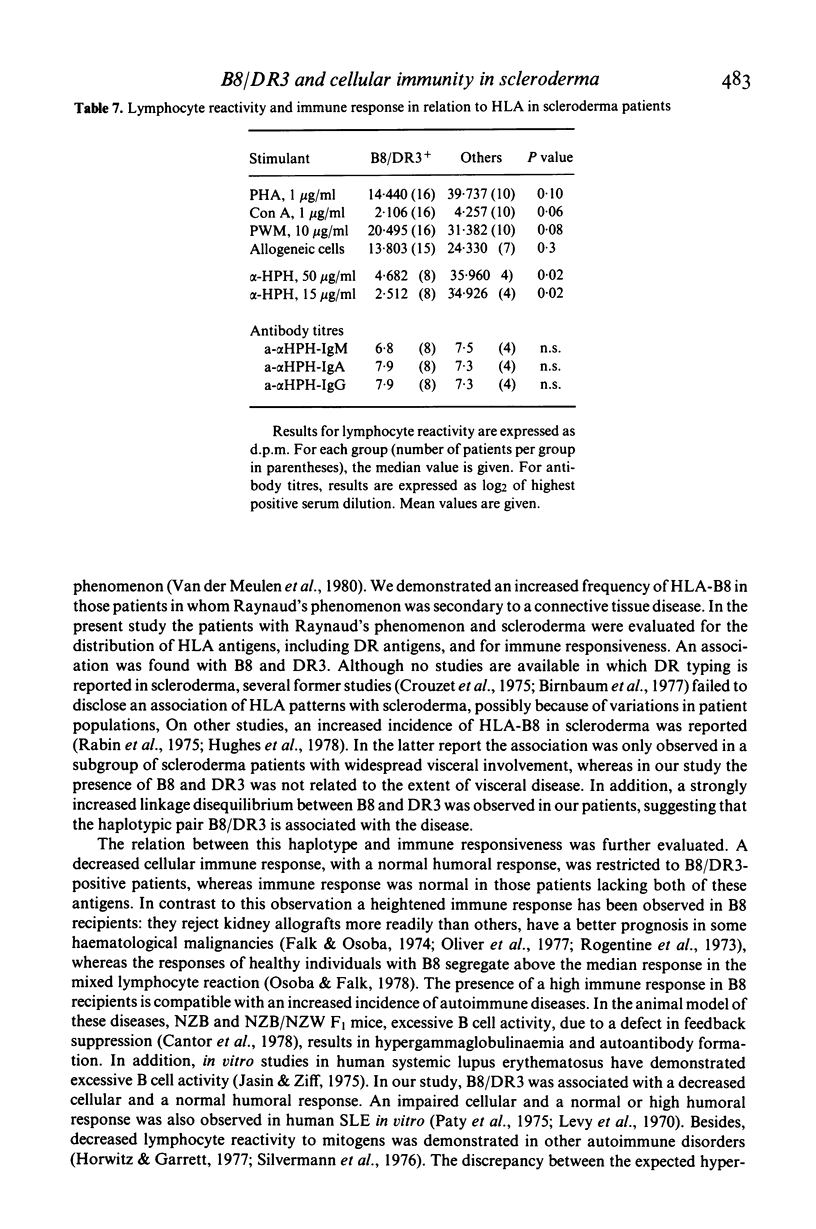
Twenty-eight patients with scleroderma were typed for 40 HLA antigens. A highly significant increase in the frequency of HLA-B8 and HLA-DR3 was observed, which was not related to the severity of the disease. In vitro lymphocyte stimulation tests were performed in 26 patients. In addition, humoral and in vitro cellular immune responses to a primary test antigen were measured in 12 of them. Although the group of scleroderma patients as a whole had an impaired cellular response, only the B8/DR3+ patients had a strongly depressed response, whereas the immune response of the others was normal. These findings suggest an association of the haplotype B8/DR3 with impaired cellular immunity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alarcón-Segovia D., Fishbein E., García-Ortigoza E., Estrada-Parra S. Uracil-specific anti-R.N.A. antibodies in scleroderma. Lancet. 1975 Feb 15;1(7903):363–366. doi: 10.1016/s0140-6736(75)91279-9. [DOI] [PubMed] [Google Scholar]
- BECK J. S., ANDERSON J. R., GRAY K. G., ROWELL N. R. ANTINUCLEAR AND PRECIPITATING AUTOANTIBODIES IN PROGRESSIVE SYSTEMIC SCLEROSIS. Lancet. 1963 Dec 7;2(7319):1188–1190. [PubMed] [Google Scholar]
- Birnbaum N. S., Rodnan G. P., Rabin B. S., Bassion S. Histocompatibility antigens in progressive systemic sclerosis (scleroderma). J Rheumatol. 1977 Winter;4(4):425–428. [PubMed] [Google Scholar]
- Cantor H., McVay-Boudreau L., Hugenberger J., Naidorf K., Shen F. W., Gershon R. K. Immunoregulatory circuits among T-cell sets. II. Physiologic role of feedback inhibition in vivo: absence in NZB mice. J Exp Med. 1978 Apr 1;147(4):1116–1125. doi: 10.1084/jem.147.4.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements P. J., Opelz G., Terasaki P. I., Mickey M. R., Furst D. Association of HLA antigen a9 with progressive systemic sclerosis (scleroderma). Tissue Antigens. 1978 Apr;11(4):357–361. doi: 10.1111/j.1399-0039.1978.tb01268.x. [DOI] [PubMed] [Google Scholar]
- Crouzet J., Marbach M. C., Camus J. P., Godeau P., Herreman G., Richier D., Hors J., Dausset J. Recherche d'une association entre antigènes HL-A et sclérodermie systémique. Nouv Presse Med. 1975 Oct 18;4(35):2489–2492. [PubMed] [Google Scholar]
- Currie S., Saunders M., Knowles M. Immunological aspects of systemic sclerosis in vitro activity of lymphocytes from patients with the disorder. Br J Dermatol. 1971 May;84(5):400–409. doi: 10.1111/j.1365-2133.1971.tb02523.x. [DOI] [PubMed] [Google Scholar]
- Dausset J., Hors J. Some contributions of the HL-A complex to the genetics of human diseases. Transplant Rev. 1975;22:44–74. doi: 10.1111/j.1600-065x.1975.tb01551.x. [DOI] [PubMed] [Google Scholar]
- HALDANE J. B. The estimation and significance of the logarithm of a ratio of frequencies. Ann Hum Genet. 1956 May;20(4):309–311. doi: 10.1111/j.1469-1809.1955.tb01285.x. [DOI] [PubMed] [Google Scholar]
- Horwitz D. A., Garrett M. A. Lymphocyte reactivity to mitogens in subjects with systemic lupus erythematosus, rheumatoid arthritis and scleroderma. Clin Exp Immunol. 1977 Jan;27(1):92–99. [PMC free article] [PubMed] [Google Scholar]
- Hughes P., Gelsthorpe K., Doughty R. W., Rowell N. R., Rosenthal F. D., Sneddon I. B. The association of HLA-B8 with visceral disease in systemic sclerosis. Clin Exp Immunol. 1978 Mar;31(3):351–356. [PMC free article] [PubMed] [Google Scholar]
- Jasin H. E., Ziff M. Immunoglobulin synthesis by peripheral blood cells in systemic lupus erythematosus. Arthritis Rheum. 1975 May-Jun;18(3):219–228. doi: 10.1002/art.1780180305. [DOI] [PubMed] [Google Scholar]
- Levy J., Barnett E. V., MacDonald N. S., Klinenberg J. R. Altered immunoglobulin metabolism in systemic lupus erythematosus and heumatoid arthritis. J Clin Invest. 1970 Apr;49(4):708–715. doi: 10.1172/JCI106283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt H. O., Bodmer W. F. Protein clinical manifestations of primary tumors of the heart. Am J Med. 1972 Jan;52(1):1–8. doi: 10.1016/0002-9343(72)90002-2. [DOI] [PubMed] [Google Scholar]
- Oliver R. T., Pillai A., Klouda P. T., Lawler S. D. HLA linked resistance factors and survival in acute myelogenous leukemia. Cancer. 1977 Jun;39(6):2337–2341. doi: 10.1002/1097-0142(197706)39:6<2337::aid-cncr2820390603>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Paty J. G., Jr, Sienknecht C. W., Townes A. S., Hanissian A. S., Miller J. B., Masi A. T. Impaired cell-mediated immunity in systemic lupus erythematosus (SLE). A controlled study of 23 untreated patients. Am J Med. 1975 Dec;59(6):769–779. doi: 10.1016/0002-9343(75)90462-3. [DOI] [PubMed] [Google Scholar]
- Rabin B. S., Rodnan G. P., Bassion S., Gill T. J., 3rd Letter: HL-A antigens in progressive systemic sclerosis (scleroderma). Arthritis Rheum. 1975 Jul-Aug;18(4):381–382. doi: 10.1002/art.1780180418. [DOI] [PubMed] [Google Scholar]
- Rogentine G. N., Trapani R. J., Yankee R. A., Henderson E. S. HL-A antigens and acute lymphocytic leukemia: the nature of the HL-A2 association. Tissue Antigens. 1973;3(6):470–476. doi: 10.1111/j.1399-0039.1973.tb00517.x. [DOI] [PubMed] [Google Scholar]
- Rothfield N. F., Rodnan G. P. Serum antinuclear antibodies in progressive systemic sclerosis (scleroderma). Arthritis Rheum. 1968 Oct;11(5):607–617. doi: 10.1002/art.1780110502. [DOI] [PubMed] [Google Scholar]
- Sasazuki T., Kohno Y., Iwamoto I., Tanimura M., Naito S. Association between an HLA haplotype and low responsiveness to tetanus toxoid in man. Nature. 1978 Mar 23;272(5651):359–361. doi: 10.1038/272359b0. [DOI] [PubMed] [Google Scholar]
- Silverman H. A., Johnson J. S., Vaughan J. H., McGlamory J. C. Altered lymphocyte reactivity in rheumatoid arthritis. Arthritis Rheum. 1976 May-Jun;19(3):509–515. doi: 10.1002/art.1780190301. [DOI] [PubMed] [Google Scholar]
- WOOLF B. On estimating the relation between blood group and disease. Ann Hum Genet. 1955 Jun;19(4):251–253. doi: 10.1111/j.1469-1809.1955.tb01348.x. [DOI] [PubMed] [Google Scholar]
- Weits J., de Gast G. C., The T. H., Esselink M. T., Deelder A. M., Petrovic M., Mandema E. Class-specific antibody titres (ELISA) against the primary immunogen Helix pomatia haemocyanin (HPH) in man. Clin Exp Immunol. 1978 Jun;32(3):443–450. [PMC free article] [PubMed] [Google Scholar]
- de Gast G. C., The T. H., Snijder J. A. The human immune response to alpha-haemocyanin of Helix pomatia. Acta Med Scand. 1973 Oct;194(4):303–309. doi: 10.1111/j.0954-6820.1973.tb19450.x. [DOI] [PubMed] [Google Scholar]
- de Vries R. P., Kreeftenberg H. G., Loggen H. G., van Rood J. J. In vitro immune responsiveness to vaccinea virus and HLA. N Engl J Med. 1977 Sep 29;297(13):692–696. doi: 10.1056/NEJM197709292971303. [DOI] [PubMed] [Google Scholar]
- van der Meulen J., van der Voort-Beelen J. M., D'Amaro J., The T. H. HLA-B8 in Raynaud's phenomenon. Tissue Antigens. 1980 Jan;15(1):81–85. doi: 10.1111/j.1399-0039.1980.tb00889.x. [DOI] [PubMed] [Google Scholar]


