Abstract
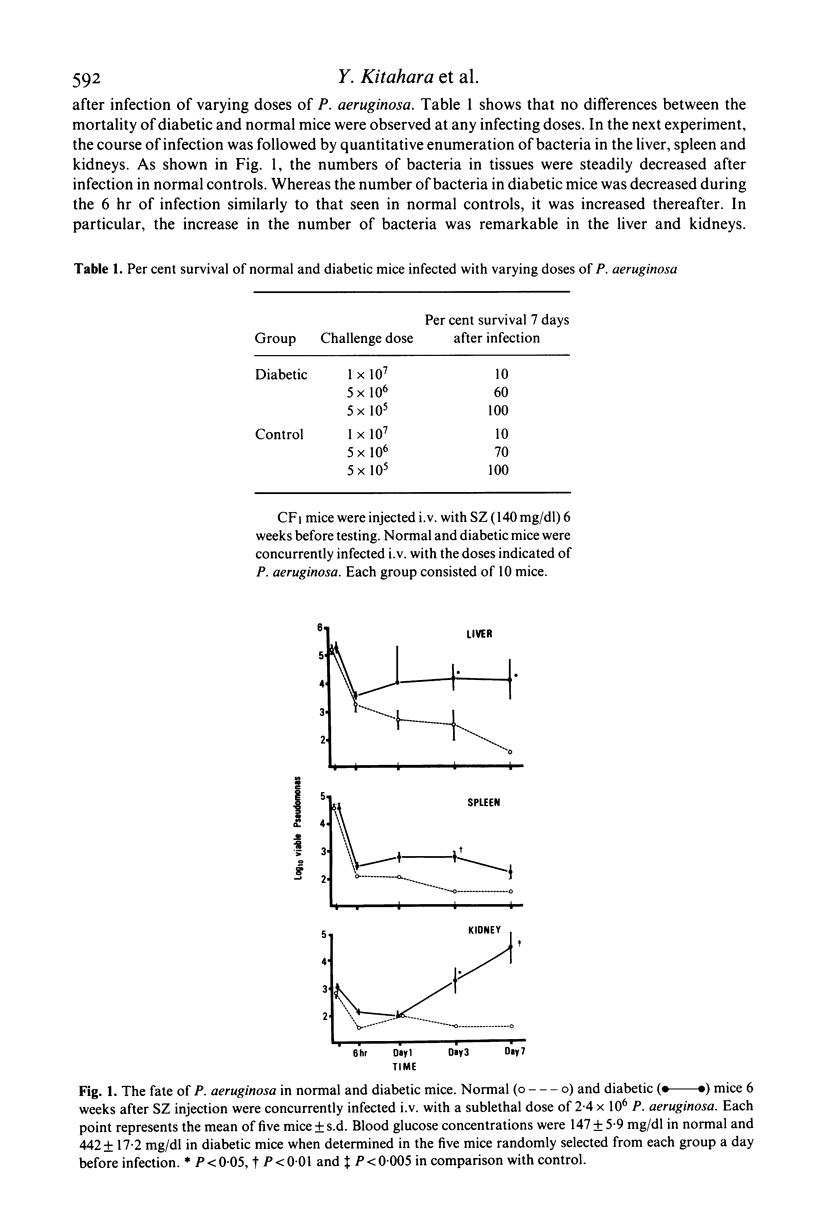
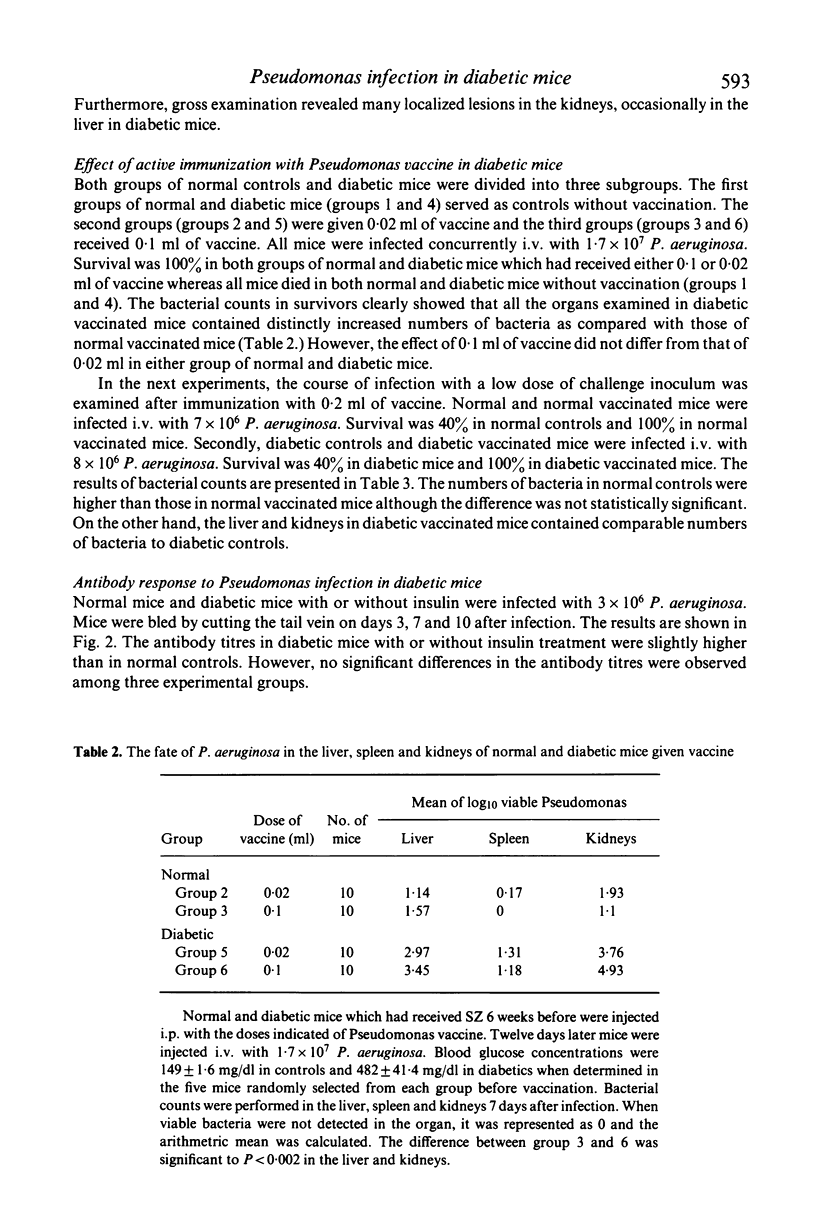
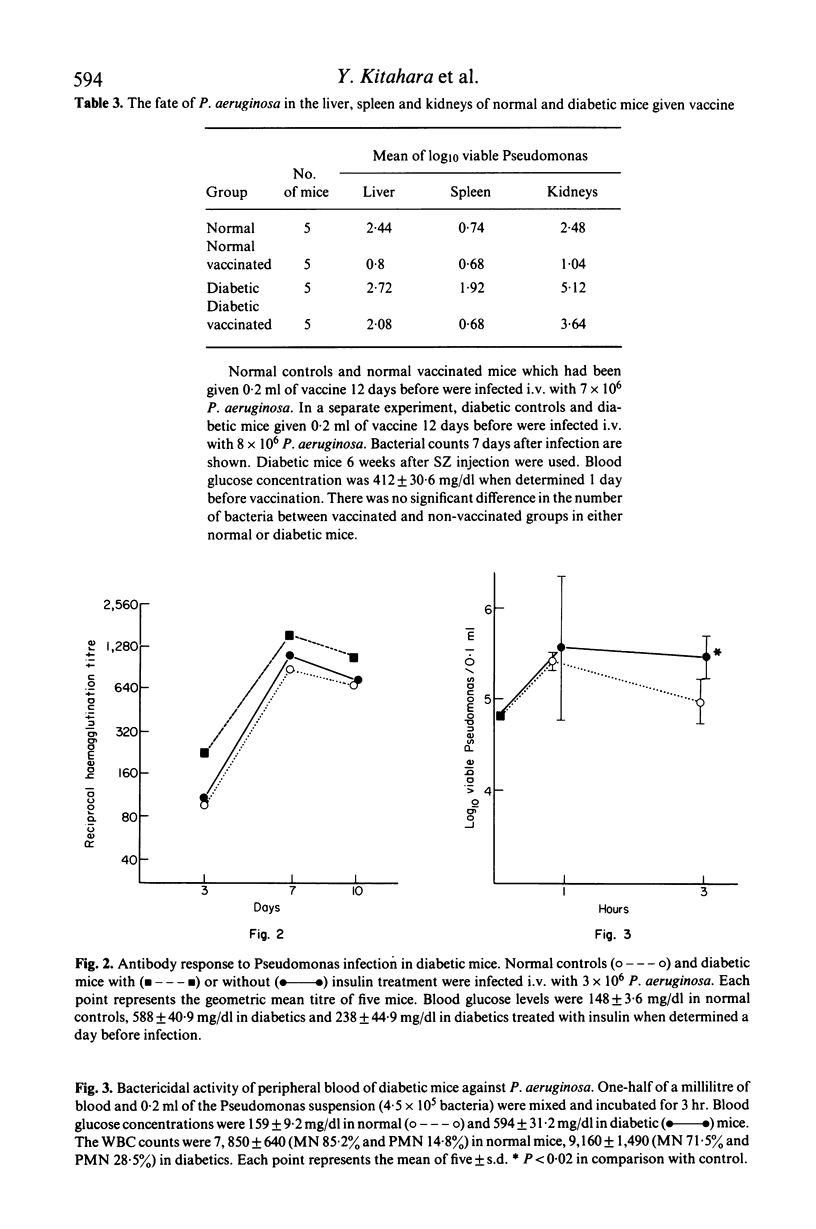
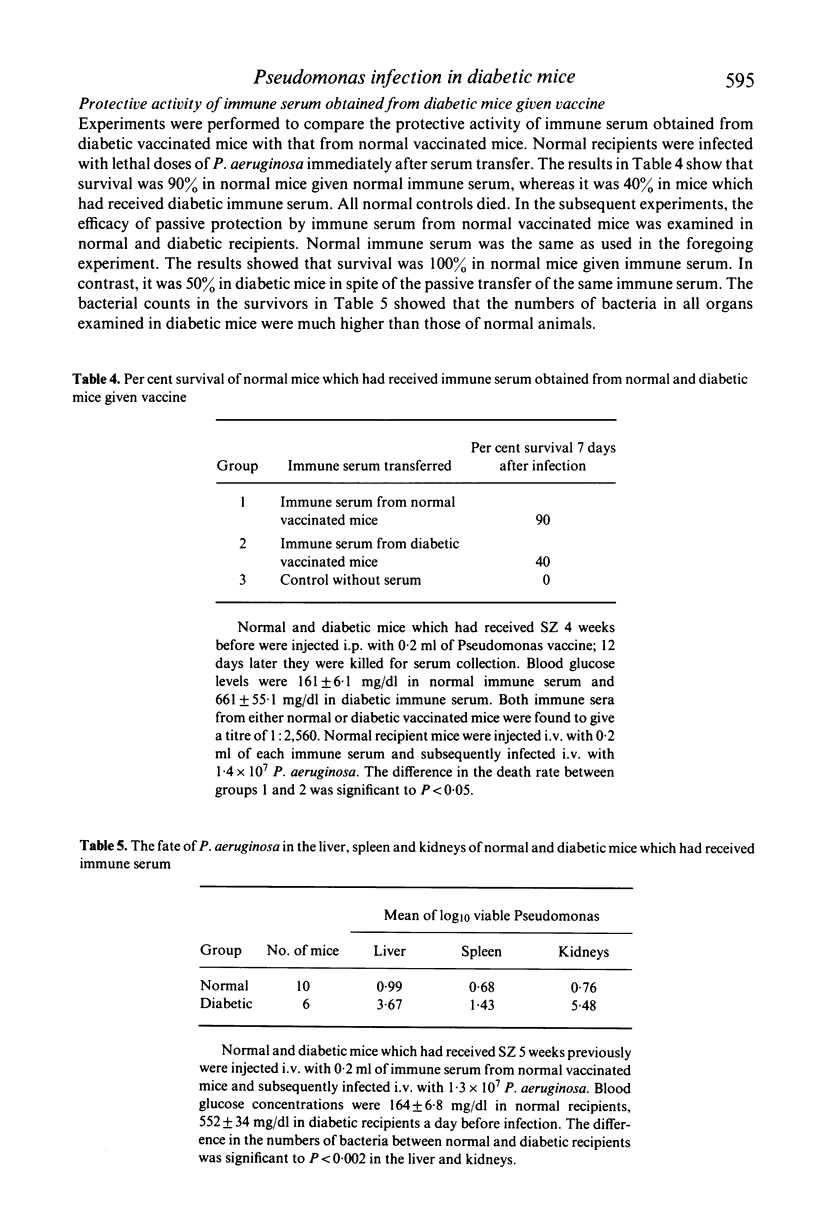
Antibacterial resistance in a diabetic state was studied using experimental Pseudomonas infection in streptozotocin (SZ) induced diabetic mice. The results obtained were as follows: (1) there was no difference in acute death rate between normal and diabetic mice when infected with Pseudomonas aeruginosa. However, a significant increase in the number of bacteria in the kidney and liver occurred at a later stage of infection in diabetic mice. (2) Active immunization with a phenolized vaccine resulted in 100% survival in either normal or diabetic mice; otherwise challenge was lethal. However, the organs examined in diabetic vaccinated mice contained distinctly increased numbers of bacteria as compared with normal vaccinated mice 7 days after infection. (3) There were no significant differences in antibody titre between normal and diabetic ice after infection, but passive protection with immune serum from diabetic vaccinated mice was less effective than that from vaccinated mice. Furthermore, immune serum from normal vaccinated mice exerted protective action less efficiently in diabetic recipients than in normal recipients. (4) The bactericidal effect of peripheral whole blood was apparently lower in diabetic mice than in normal mice. (5) Treatment with insulin restored such reduced resistance to Pseudomonas infection in diabetic mice. These findings suggest that the decreased resistance to Pseudomonas infection in diabetic mice should be ascribed to impaired function of antibody, abnormalities in phagocytic cells and disturbed microcirculation caused by the insulin-deficient state.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BYBEE J. D., ROGERS D. E. THE PHAGOCYTIC ACTIVITY OF POLYMORPHONUCLEAR LEUKOCYTES OBTAINED FROM PATIENTS WITH DIABETES MELLITUS. J Lab Clin Med. 1964 Jul;64:1–13. [PubMed] [Google Scholar]
- Bagdade J. D., Nielson K. L., Bulger R. J. Reversible abnormalities in phagocytic function in poorly controlled diabetic patients. Am J Med Sci. 1972 Jun;263(6):451–456. doi: 10.1097/00000441-197206000-00005. [DOI] [PubMed] [Google Scholar]
- Bar R. S., Kahn C. R., Koren H. S. Insulin inhibition of antibody-dependent cytoxicity and insulin receptors in macrophages. Nature. 1977 Feb 17;265(5595):632–635. doi: 10.1038/265632a0. [DOI] [PubMed] [Google Scholar]
- Bartell P. F., Orr T. E., Garcia M. The lethal events in experimental Pseudomonas aeruginosa infection of mice. J Infect Dis. 1968 Apr;118(2):165–172. doi: 10.1093/infdis/118.2.165. [DOI] [PubMed] [Google Scholar]
- Bennett J. V. Nosocomial infections due to Pseudomonas. J Infect Dis. 1974 Nov;130 (Suppl)(0):S4–S7. doi: 10.1093/infdis/130.supplement.s4. [DOI] [PubMed] [Google Scholar]
- Bjornson A. B., Michael J. G. Biological Activities of Rabbit Immunoglobulin M and Immunoglobulin G Antibodies to Pseudomonas aeruginosa. Infect Immun. 1970 Oct;2(4):453–461. doi: 10.1128/iai.2.4.453-461.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson A. B., Michael J. G. Contribution of humoral and cellular factors to the resistance to experimental infection by Pseudomonas aeruginosa in mice. I. Interaction between immunoglobulins, heat-labile serum factors, and phagocytic cells in the killing of bacteria. Infect Immun. 1971 Oct;4(4):462–467. doi: 10.1128/iai.4.4.462-467.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson A. B., Michael J. G. Contribution of humoral and cellular factors to the resistance to experimental infection by Pseudomonas aeruginosa in mice. II. Opsonic, agglutinative, and protective capacities of immunoglobulin G anti-Pseudomonas antibodies. Infect Immun. 1972 May;5(5):775–782. doi: 10.1128/iai.5.5.775-782.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finland M. Excursions into epidemiology: selected studies during the past four decades at Boston City Hospital. J Infect Dis. 1973 Jul;128(1):76–124. doi: 10.1093/infdis/128.1.76. [DOI] [PubMed] [Google Scholar]
- FitzPatrick F. K., Girard A. E. Pyelonephritis in the mouse. II. Vaccination studies. Proc Soc Exp Biol Med. 1968 Feb;127(2):579–585. doi: 10.3181/00379727-127-32746. [DOI] [PubMed] [Google Scholar]
- Fussganger R. D., Kahn C. R., Roth J., De Meyts P. Binding and degradation of insulin by human peripheral granulocytes. Demonstration of specific receptors with high affinity. J Biol Chem. 1976 May 10;251(9):2761–2769. [PubMed] [Google Scholar]
- GORRILL R. H. THE FATE OF PSEUDOMONAS AERUGINOSA, PROTEUS MIRABILIS AND ESCHERICHIA COLI IN THE MOUSE KIDNEY. J Pathol Bacteriol. 1965 Jan;89:81–88. doi: 10.1002/path.1700890110. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Buell D. N., Roth J. Water-soluble insulin receptors from human lymphocytes. Science. 1972 Oct 13;178(4057):168–169. doi: 10.1126/science.178.4057.168. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Gorden P., Roth J., Archer J. A., Buell D. N. Characteristics of the human lymphocyte insulin receptor. J Biol Chem. 1973 Mar 25;248(6):2202–2207. [PubMed] [Google Scholar]
- Harado S., Ishibashi T., Kitahara Y., Harada Y., Takamoto M., Sugiyama K. Experimental Pseudomonas infection in mice: effect of single cyclophosphamide administration on Pseudomonas infection. Jpn J Exp Med. 1979 Feb;49(1):43–50. [PubMed] [Google Scholar]
- Homma J. Y. Recent investigations on Pseudomonas aeruginosa. Jpn J Exp Med. 1971 Oct;41(5):387–400. [PubMed] [Google Scholar]
- Ishibashi T., Harada S., Harada Y., Kitahara Y., Takamoto M., Sugiyama K. Experimental Pseudomonas infection in mice: acquired resistance against Pseudomonas septicemia and altered susceptibility in BCG infected mice. Jpn J Exp Med. 1978 Aug;48(4):313–320. [PubMed] [Google Scholar]
- Ishibashi T., Kitahara Y., Harada Y., Harada S., Takamoto M., Ishibashi T. Immunologic features of mice with streptozotocin-induced diabetes: depression of their immune responses to sheep red blood cells. Diabetes. 1980 Jul;29(7):516–523. doi: 10.2337/diab.29.7.516. [DOI] [PubMed] [Google Scholar]
- Jones R. J., Lilly H. A., Lowbury E. J. Passive protection of mice against Pseudomonas aeruginosa by serum from recently vaccinated mice. Br J Exp Pathol. 1971 Jun;52(3):264–270. [PMC free article] [PubMed] [Google Scholar]
- Jones R. J. Protection against Pseudomonas aeruginosa infection by immunisation with fractions of culture filtrates of Ps. aeruginosa. Br J Exp Pathol. 1968 Oct;49(5):411–420. [PMC free article] [PubMed] [Google Scholar]
- Kobayashi F. Experimental infection with Pseudomonas aeruginosa in mice. II. The fate of highly and low virulent strains in the peritoneal cavity and organs of mice. Jpn J Microbiol. 1971 Jul;15(4):301–307. [PubMed] [Google Scholar]
- Lundboek K., Olsen T. S., Orskov H., Hansen R. O. Long-term experimental insulin-deficiency diabetes--a model of diabetic angiopathy? Acta Med Scand Suppl. 1967;476:159–173. doi: 10.1111/j.0954-6820.1967.tb12694.x. [DOI] [PubMed] [Google Scholar]
- MILLICAN R. C., RUST J. D. Efficacy of rabbit pseudomonas antiserum in experimental Pseudomonas aeruginosa infection. J Infect Dis. 1960 Nov-Dec;107:389–394. doi: 10.1093/infdis/107.3.389. [DOI] [PubMed] [Google Scholar]
- Mowat A., Baum J. Chemotaxis of polymorphonuclear leukocytes from patients with diabetes mellitus. N Engl J Med. 1971 Mar 25;284(12):621–627. doi: 10.1056/NEJM197103252841201. [DOI] [PubMed] [Google Scholar]
- Nolan C. M., Beaty H. N., Bagdade J. D. Further characterization of the impaired bactericidal function of granulocytes in patients with poorly controlled diabetes. Diabetes. 1978 Sep;27(9):889–894. doi: 10.2337/diab.27.9.889. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Reaven G. M. Insulin binding to monocytes and total mononuclear leukocytes from normal and diabetic patients. J Clin Endocrinol Metab. 1976 Jul;43(1):226–231. doi: 10.1210/jcem-43-1-226. [DOI] [PubMed] [Google Scholar]
- PERILLIE P. E., NOLAN J. P., FINCH S. C. Studies of the resistance to infection in diabetes mellitus: local exudative cellular response. J Lab Clin Med. 1962 Jun;59:1008–1015. [PubMed] [Google Scholar]
- Peto R. Editorial: Guidelines on the analysis of tumour rates and death rates in experimental animals. Br J Cancer. 1974 Feb;29(2):101–105. doi: 10.1038/bjc.1974.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson C. L., Johnson A. G., Feller I. Effect of cyclophosphamide on the immune response to Pseudomonas aeruginosa in mice. Infect Immun. 1976 Jul;14(1):168–177. doi: 10.1128/iai.14.1.168-177.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. Y., Kazmierowski J. A., Newball H. H. Specificity of opsonic antibodies to enhance phagocytosis of Pseudomonas aeruginosa by human alveolar macrophages. J Clin Invest. 1975 Aug;56(2):376–385. doi: 10.1172/JCI108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. Modulation of macrophage Fc receptor expression in vitro by insulin and cyclic nucleotides. Nature. 1975 Oct 16;257(5527):597–599. doi: 10.1038/257597a0. [DOI] [PubMed] [Google Scholar]
- SHELDON W. H., BAUER H. The development of the acute inflammatory response to experimental cutaneous mucormycosis in normal and diabetic rabbits. J Exp Med. 1959 Dec 1;110:845–852. doi: 10.1084/jem.110.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H., Bianco A. R., Handwerger B. S., Kahn C. R. Demonstration that monocytes rather than lymphocytes are the insulin-binding cells in preparations of humah peripheral blood mononuclear leukocytes: implications for studies of insulin-resistant states in man. Proc Natl Acad Sci U S A. 1975 Feb;72(2):474–478. doi: 10.1073/pnas.72.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom T. B., Bear R. A., Carpenter C. B. Insulin-induced augmentation of lymphocyte-mediated cytotoxicity. Science. 1975 Mar 28;187(4182):1206–1208. doi: 10.1126/science.163492. [DOI] [PubMed] [Google Scholar]
- Tomiyama T., Homma J. Y., Abe C., Yoichi M. Passive hemagglutination reaction using formalinized sheep erythrocytes treated with tannin and coated with protein moiety of the endotoxin (OEP) of Pseudomonas aeruginosa. Jpn J Exp Med. 1973 Jun;43(3):185–189. [PubMed] [Google Scholar]
- WERTMAN K. F., HENNEY M. R. The effects of alloxan diabetes on phagocytosis and susceptibility to infection. J Immunol. 1962 Sep;89:314–317. [PubMed] [Google Scholar]
- Young L. S., Armstrong D. Human immunity to Pseudomonas aeruginosa. I. In-vitro interaction of bacteria, polymorphonuclear leukocytes, and serum factors. J Infect Dis. 1972 Sep;126(3):257–276. doi: 10.1093/infdis/126.3.257. [DOI] [PubMed] [Google Scholar]


