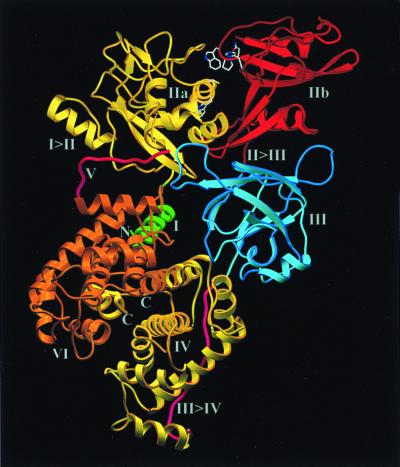
Figure 1.
Ribbon structure of human m-calpain in the absence of calcium, shown in reference orientation. The 80-kDa L-chain starts in the molecular center (green, dI), folds into the surface of the dIIa subdomain (gold, I→II linker), forms the papain-like left-side part of the catalytic domain dII (gold, dIIa) and the right-side barrel-like subdomain dIIb (red), descends through the open II→III loop (red), builds domain dIII (blue), runs down (magenta, III→IV), and forms the right-side calmodulin-like domain dIV (yellow). The 30-kDa S-chain becomes visible from Thr95S onwards (magenta, dV) before forming the left-side calmodulin domain dVI (orange). The catalytic residues Cys105L, His262L, and Asn286L together with Trp106L, Pro287L, and Trp288L (top) are shown with all non-hydrogen atoms. The figure was made with setor (34).