Abstract
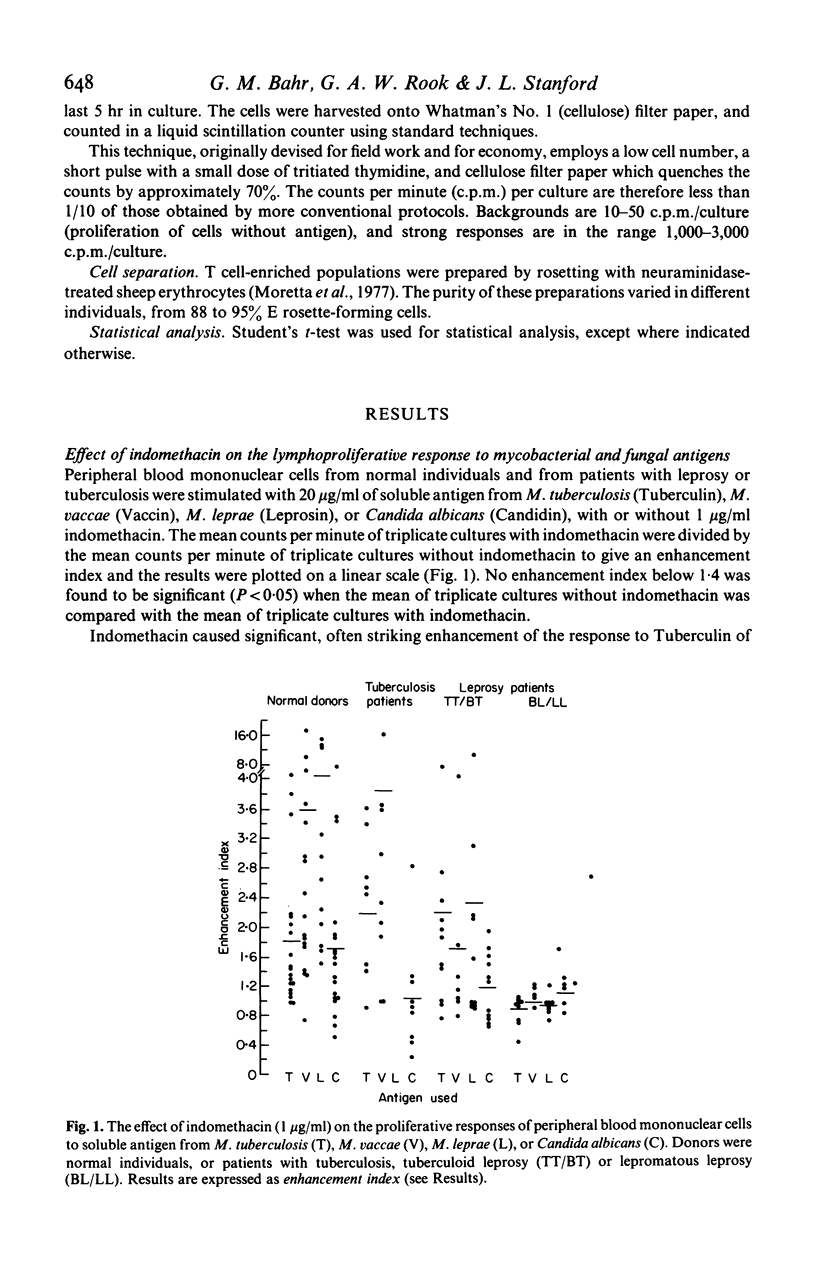
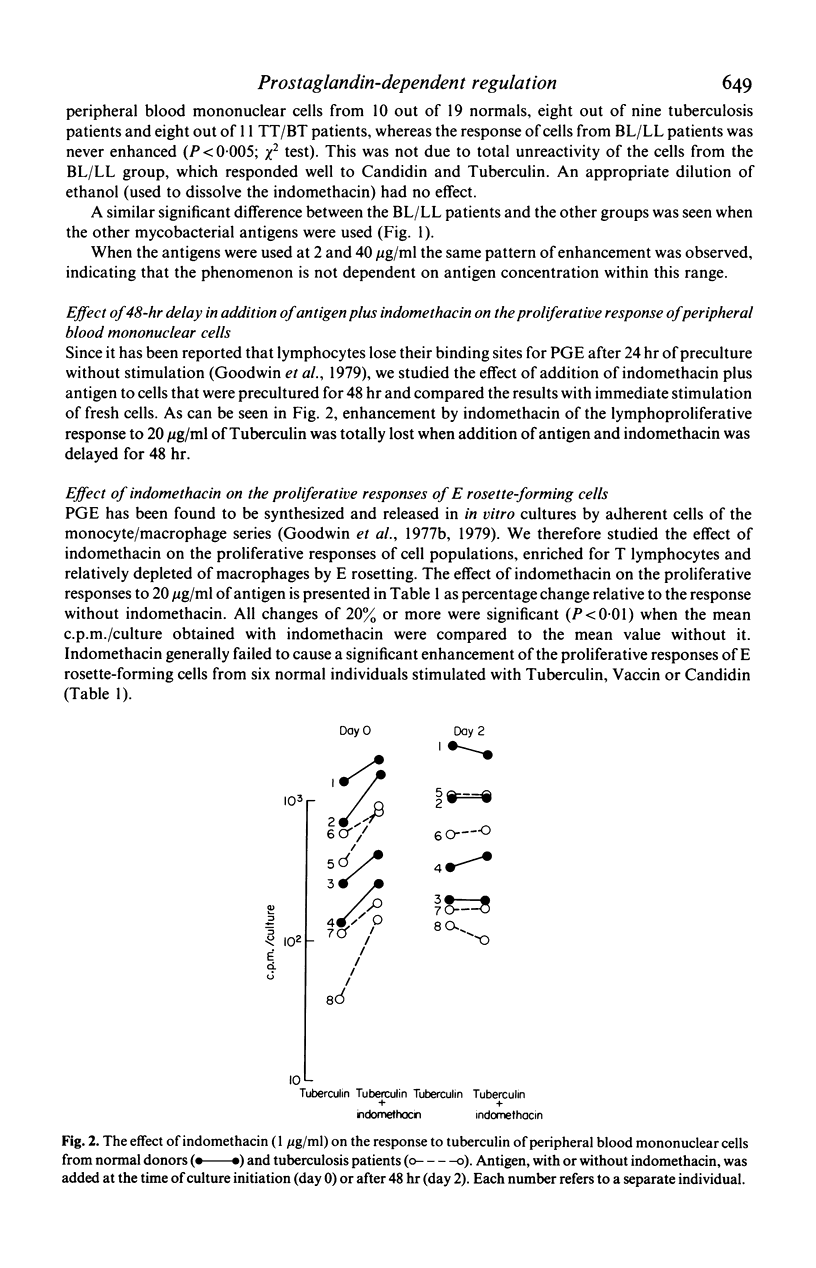
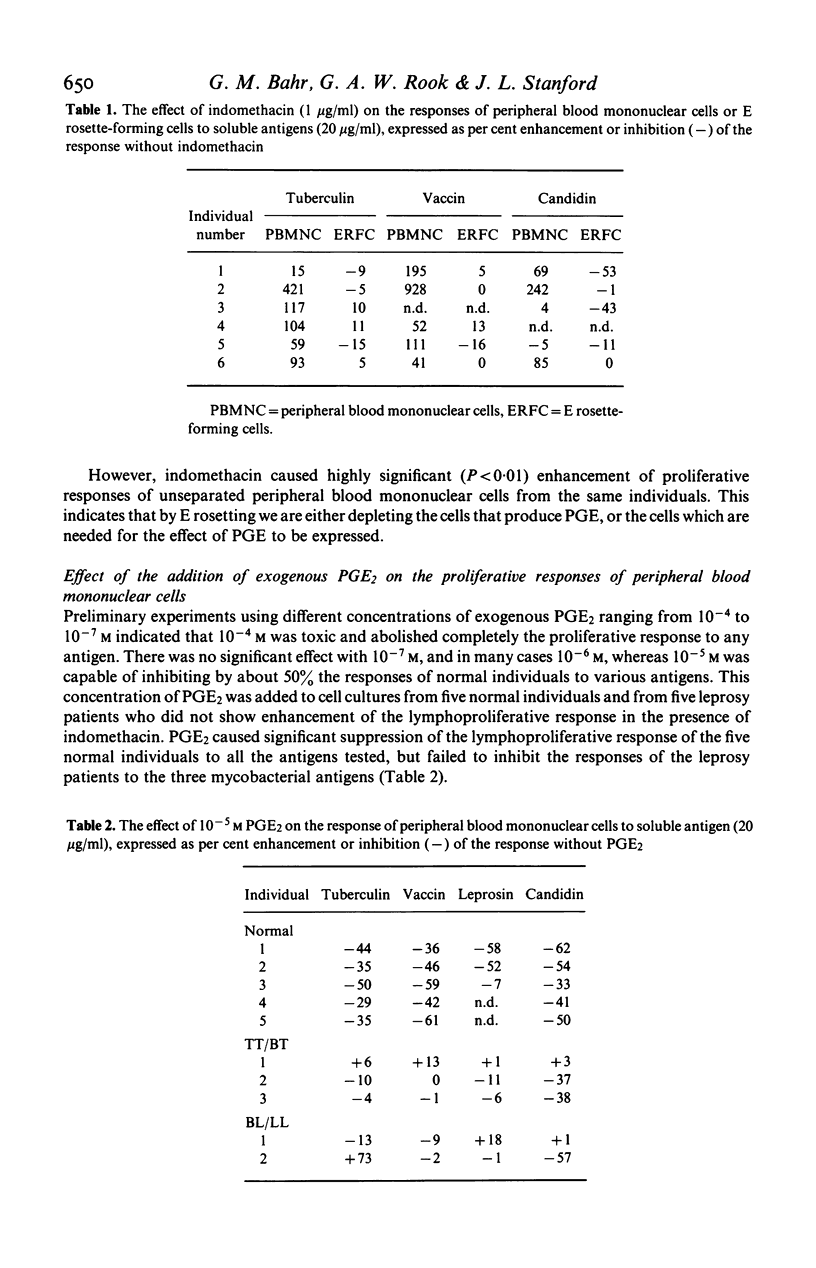
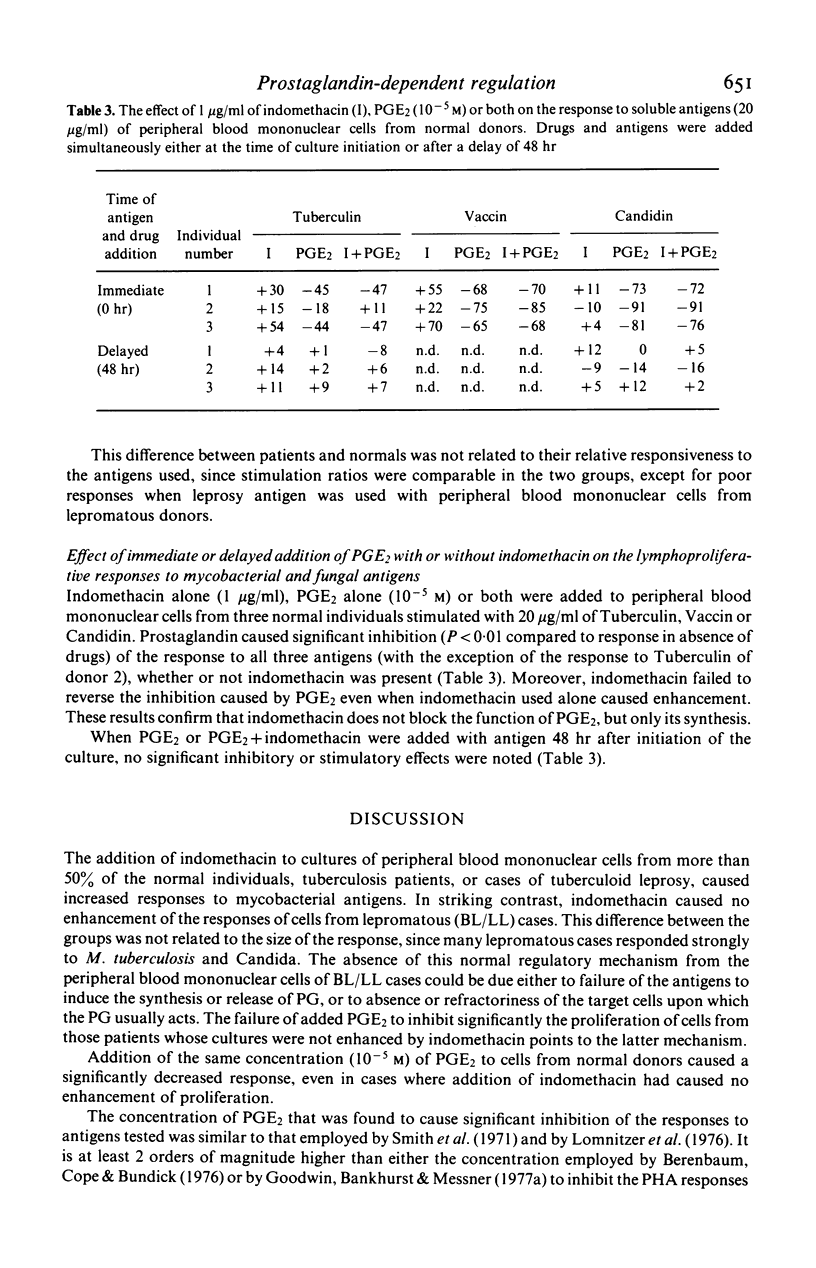
The response to soluble mycobacterial antigens of peripheral blood mononuclear cells, from most normal donors, tuberculosis patients or cases of tuberculoid leprosy (TT/BT) was enhanced by the addition of indomethacin. In contrast, indomethacin caused no enhancement of the response of cells from lepromatous leprosy (BL/LL) cases. Moreover the addition of 10-5 M prostaglandin E2 (PGE2) failed to inhibit the proliferative responses of cells from the BL/LL patients, although it markedly inhibited the responses of peripheral blood mononuclear cells from the other groups. The addition of PGE2 or indomethacin to cells which had been precultured for 48 hr had no significant effect on the proliferative responses of cells from any of the groups of donors. These results suggest that a normal, prostaglandin-dependent, indomethacin-sensitive regulatory mechanism is absent from the peripheral blood mononuclear cells of BL/LL patients.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berenbaum M. C., Cope W. A., Bundick R. V. Synergistic effect of cortisol and prostaglandin E2 on the PHA response. Relation to immunosuppression induced by trauma. Clin Exp Immunol. 1976 Dec;26(3):534–541. [PMC free article] [PubMed] [Google Scholar]
- Bona C., Audibert F., Juy D., Chedid L. Cell suppression in PPD-induced blast specific response of human peripheral blood lymphocytes. Clin Exp Immunol. 1976 Nov;26(2):258–266. [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R., Lichtenstein L. M., Melmon K. L., Henney C. S., Weinstein Y., Shearer G. M. Modulation of inflammation and immunity by cyclic AMP. Science. 1974 Apr 5;184(4132):19–28. doi: 10.1126/science.184.4132.19. [DOI] [PubMed] [Google Scholar]
- Ellner J. J., Spagnuolo P. J. Suppression of antigen and mitogen induced human T lymphocyte DNA synthesis by bacterial lipopolysaccharide: mediation by monocyte activation and production of prostaglandins. J Immunol. 1979 Dec;123(6):2689–2695. [PubMed] [Google Scholar]
- Ellner J. J. Suppressor adherent cells in human tuberculosis. J Immunol. 1978 Dec;121(6):2573–2579. [PubMed] [Google Scholar]
- Ferraris V. A., DeRubertis F. R. Release of prostaglandin by mitogen- and antigen-stimulated leukocytes in culture. J Clin Invest. 1974 Aug;54(2):378–386. doi: 10.1172/JCI107773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Bankhurst A. D., Messner R. P. Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J Exp Med. 1977 Dec 1;146(6):1719–1734. doi: 10.1084/jem.146.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Wiik A., Lewis M., Bankhurst A. D., Williams R. C., Jr High-affinity binding sites for prostaglandin E on human lymphocytes. Cell Immunol. 1979 Mar 1;43(1):150–159. doi: 10.1016/0008-8749(79)90158-8. [DOI] [PubMed] [Google Scholar]
- Laughter A. H., Twomey J. J. Suppression of lymphoproliferation by high concentrations of normal human mononuclear leukocytes. J Immunol. 1977 Jul;119(1):173–179. [PubMed] [Google Scholar]
- Lomnitzer R., Rabson A. R., Koornhof H. J. The effects of cyclic AMP on leucocyte inhibitory factor (LIF) production and on the inhibition of leucocyte migration. Clin Exp Immunol. 1976 Apr;24(1):42–48. [PMC free article] [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrvang B., Godal T., Ridley D. S., Fröland S. S., Song Y. K. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum of leprosy. Clin Exp Immunol. 1973 Aug;14(4):541–553. [PMC free article] [PubMed] [Google Scholar]
- Nath I., Van Rood J. J., Mehra N. K., Vaidya M. C. Natural suppressor cells in human leprosy: the role of HLA-D-identical peripheral lymphocytes and macrophages in the in vitro modulation of lymphoproliferative responses. Clin Exp Immunol. 1980 Nov;42(2):203–210. [PMC free article] [PubMed] [Google Scholar]
- Paul R. C., Stanford J. L., Carswell J. W. Multiple skin testing in leprosy. J Hyg (Lond) 1975 Aug;75(1):57–68. doi: 10.1017/s0022172400047069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice L., Laughter A. H., Twomey J. J. Three suppressor systems in human blood that modulate lymphoproliferation. J Immunol. 1979 Mar;122(3):991–996. [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Rook G. A., Carswell J. W., Stanford J. L. Preliminary evidence for the trapping of antigen-specific lymphocytes in the lymphoid tissue of 'anergic' tuberculosis patients. Clin Exp Immunol. 1976 Oct;26(1):129–132. [PMC free article] [PubMed] [Google Scholar]
- Shou L., Schwartz S. A., Good R. A. Suppressor cell activity after concanavalin A treatment of lymphocytes from normal donors. J Exp Med. 1976 May 1;143(5):1100–1110. doi: 10.1084/jem.143.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Steiner A. L., Parker C. W. Human lymphocytic metabolism. Effects of cyclic and noncyclic nucleotides on stimulation by phytohemagglutinin. J Clin Invest. 1971 Feb;50(2):442–448. doi: 10.1172/JCI106511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk J. L., Bryceson A. D. Immunological phenomena in leprosy and related diseases. Adv Immunol. 1971;13:209–266. doi: 10.1016/s0065-2776(08)60185-6. [DOI] [PubMed] [Google Scholar]
- Webb D. R., Nowowiejski I. Mitogen-induced changes in lymphocyte prostaglandin levels: a signal for the induction of suppressor cell activity. Cell Immunol. 1978 Nov;41(1):72–85. doi: 10.1016/s0008-8749(78)80029-x. [DOI] [PubMed] [Google Scholar]


