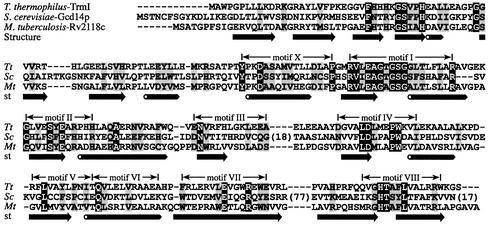
Figure 1.
Sequence alignment of S.cerevisiae Gcd14p, M.tuberculosis Rv2118c and their T.thermophilus ortholog (TrmI). The size of insertions in Gcd14p omitted for clarity is indicated in parentheses. Highly conserved residues are shown on a black background and residues with a similar physico-chemical character are on a gray background. The experimentally determined secondary structure of M.tuberculosis Rv2118c is shown below its sequence as arrows (strands) and tubes (helices). Within the catalytic domain (central and C-terminal parts of the protein), the nine sequence motifs typical for the ‘classical’ AdoMet-dependent MTase family (14) are indicated.