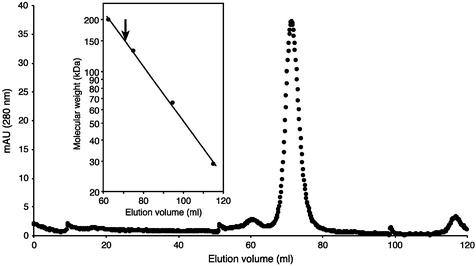
Figure 4.
Estimation of the apparent molecular weight of the purified His-tagged T.thermophilus TrmI protein by gel filtration chromatography on a Superdex 200 prep grade 16/60 column (Pharmacia Biotech). The sample consisted of 2.5 mg protein in 50 mM Tris–HCl pH 8.5, 500 mM KCl, 200 mM imidazole. Elution was performed with the same buffer. (Inset) Calculation of the apparent molecular weight of the T.thermophilus TrmI protein. The standard consisted of carbonic anhydrase from bovine erythrocytes (29 kDa), bovine serum albumin (66 kDa), bovine serum albumin dimer (132 kDa) and β-amylase from sweet potato (200 kDa). The elution volume corresponding to the T.thermophilus TrmI protein is indicated by an arrow.