Abstract
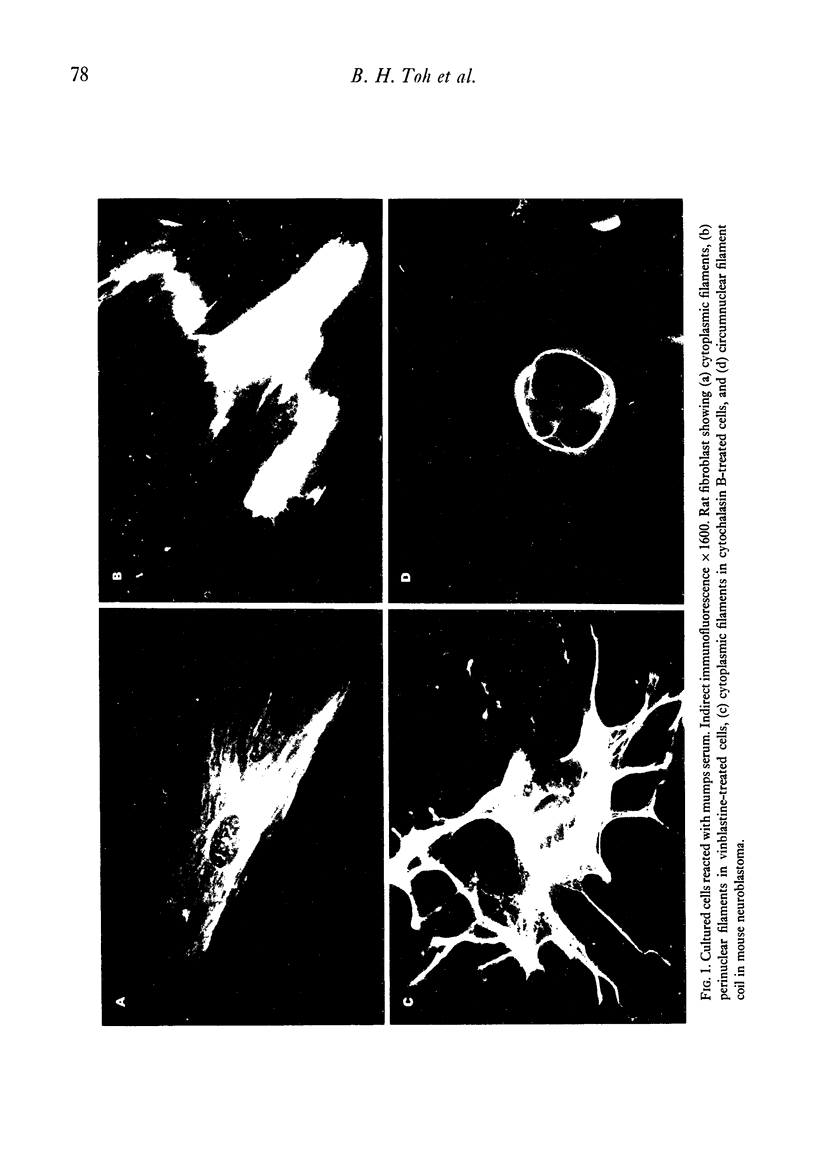
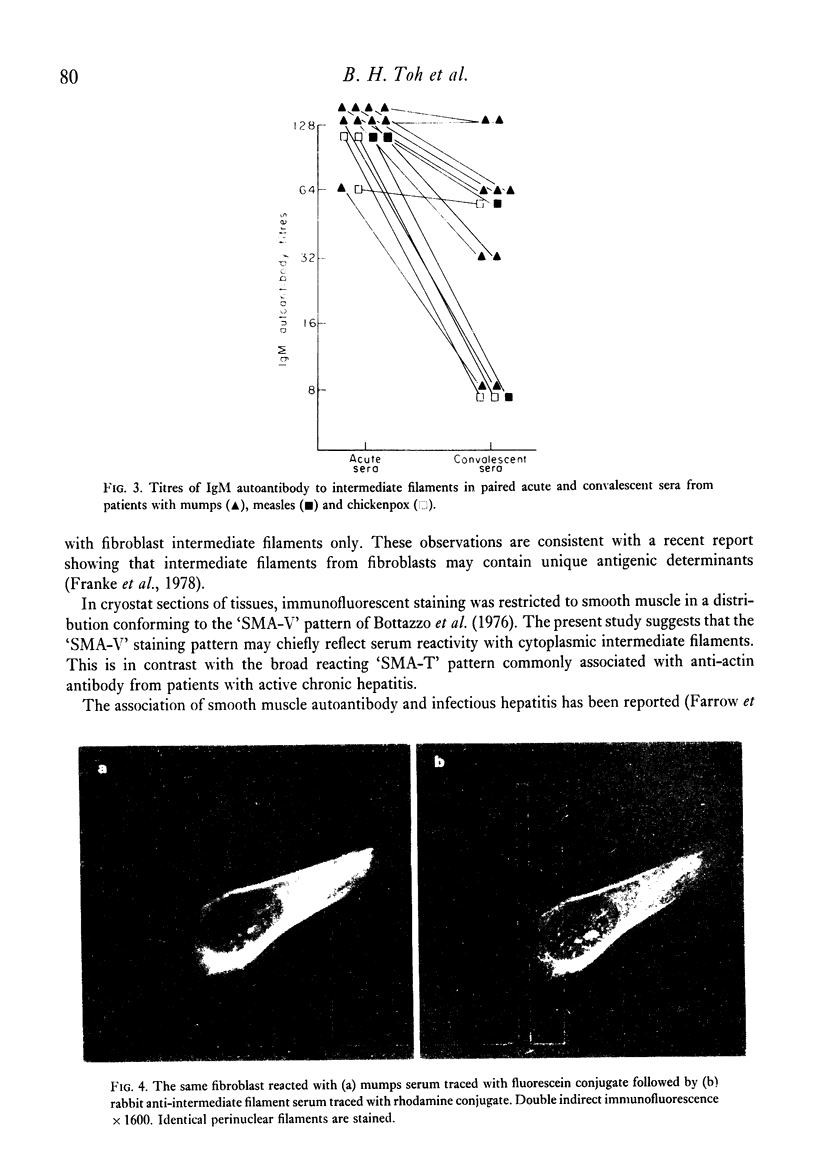
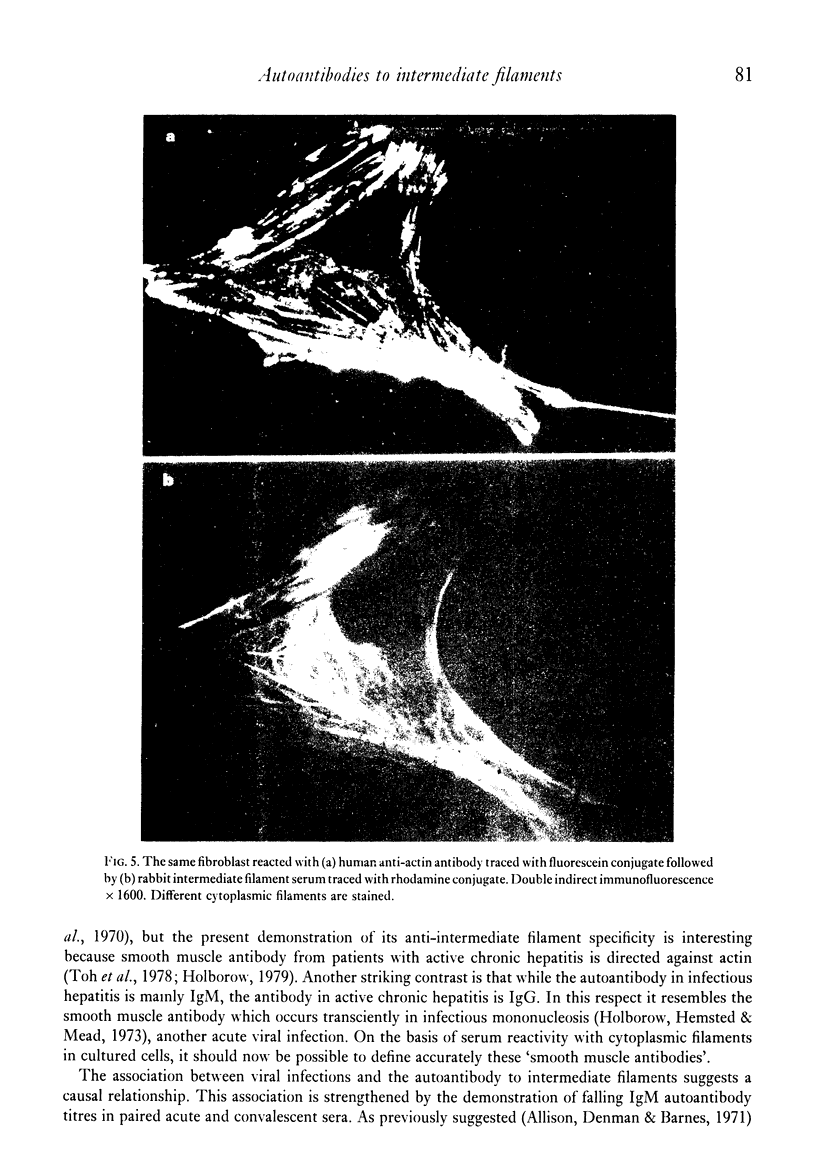
Seventy-four out of 113 sera from patients with infectious hepatitis, chickenpox, measles and mumps reacted with both smooth muscle and cytoplasmic filaments in cultured fibroblasts and neuroblastoma. Five out of eighty-five control sera also reacted in this way. That the cytoplasmic structures are intermediate filaments was suggested by their rearrangement into coils of perinuclear filaments in colchicine- or vinblastine-treated fibroblasts, but not in cytochalasin B-treated cells. The idenity of these structures was confirmed by the demonstration that the same structures reacted with the post-viral sera and a rabbit and human anti-intermediate filament antibody. Immunoabsorption studies showed that twenty-seven out of thirty-two positive sera were neutralised by skeletin, the intermediate filament protein from smooth muscle. In all but one of the sera, the antibody was IgM. Antibody titres fell in the second specimen in eleven out of fourteen pairs of acute and convalescent sera. The association between viral infections and autoantibodies suggest that production of antibodies suggests that production of antibody to intermediate filaments may be initiated by viruses.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Denman A. M., Barnes R. D. Cooperating and controlling functions of thymus-derived lymphocytes in relation to autoimmunity. Lancet. 1971 Jul 17;2(7716):135–140. doi: 10.1016/s0140-6736(71)92306-3. [DOI] [PubMed] [Google Scholar]
- Bottazzo G. F., Florin-Christensen A., Fairfax A., Swana G., Doniach D., Groeschel-Stewart U. Classification of smooth muscle autoantibodies detected by immunofluorescence. J Clin Pathol. 1976 May;29(5):403–410. doi: 10.1136/jcp.29.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow L. J., Holborow E. J., Johnson G. D., Lamb S. G., Stewart J. S., Taylor P. E., Zuckerman A. J. Autoantibodies and the hepatitis-associated antigen in acute infective hepatitis. Br Med J. 1970 Jun 20;2(5711):693–695. doi: 10.1136/bmj.2.5711.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon W. E., 3rd, Bushnell A., Burridge K. Characterization of the intermediate (10 nm) filaments of cultured cells using an autoimmune rabbit antiserum. Cell. 1978 Feb;13(2):249–261. doi: 10.1016/0092-8674(78)90194-0. [DOI] [PubMed] [Google Scholar]
- Haire M. Fibrillar anti-cellular antibody associated with mumps and measles infection. Clin Exp Immunol. 1972 Nov;12(3):335–341. [PMC free article] [PubMed] [Google Scholar]
- Holborow E. J., Hemsted E. H., Mead S. V. Smooth muscle autoantibodies in infectious mononucleosis. Br Med J. 1973 Aug 11;3(5875):323–325. doi: 10.1136/bmj.3.5875.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen A. O., Subrahmanyan L., Turnbull C., Kalnins V. I. Localization of the neurofilament protein in neuroblastoma cells by immunofluorescent staining. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3192–3196. doi: 10.1073/pnas.73.9.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanakoudi F., Nikolaidis A., Daniilidis B., Manios S., Zurukzoglu S. S., Cassimos C. Immunological studies in children with acute viral hepatitis. Clin Exp Immunol. 1975 Oct;22(1):78–83. [PMC free article] [PubMed] [Google Scholar]
- Kurki P., Linder E., Virtanen I., Stenman S. Human smooth muscle autoantibodies reacting with intermediate (100 A) filaments. Nature. 1977 Jul 21;268(5617):240–241. doi: 10.1038/268240a0. [DOI] [PubMed] [Google Scholar]
- Mousa G. Y., Trevithick J. R., Bechberger J., Blair D. G. Cytochalasin D induces the capping of both leukaemia viral proteins and actin in infected cells. Nature. 1978 Aug 24;274(5673):808–809. doi: 10.1038/274808a0. [DOI] [PubMed] [Google Scholar]
- Rose N. R. Autoimmunity revisited. Nature. 1978 Sep 14;275(5676):88–90. doi: 10.1038/275088a0. [DOI] [PubMed] [Google Scholar]
- Small J. V., Sobieszek A. Studies on the function and composition of the 10-NM(100-A) filaments of vertebrate smooth muscle. J Cell Sci. 1977 Feb;23:243–268. doi: 10.1242/jcs.23.1.243. [DOI] [PubMed] [Google Scholar]
- Starger J. M., Goldman R. D. Isolation and preliminary characterization of 10-nm filaments from baby hamster kidney (BHK-21) cells. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2422–2426. doi: 10.1073/pnas.74.6.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh B. H., Clarke F. M., Ceredig R. Reaction of human smooth muscle autoantibody with skeletal muscle, cardiac muscle, and thymic myoid cells. Clin Immunol Immunopathol. 1978 Jan;9(1):28–36. doi: 10.1016/0090-1229(78)90117-4. [DOI] [PubMed] [Google Scholar]
- Toh B. H., Gallichio H. A., Jeffrey P. L., Livett B. G., Muller H. K., Cauchi M. N., Clarke F. M. Anti-actin stains synapses. Nature. 1976 Dec 16;264(5587):648–650. doi: 10.1038/264648a0. [DOI] [PubMed] [Google Scholar]
- Toh B. H., Hard C. C. Actin co-caps with concanavalin A receptors. Nature. 1977 Oct 20;269(5630):695–697. doi: 10.1038/269695a0. [DOI] [PubMed] [Google Scholar]


