Abstract
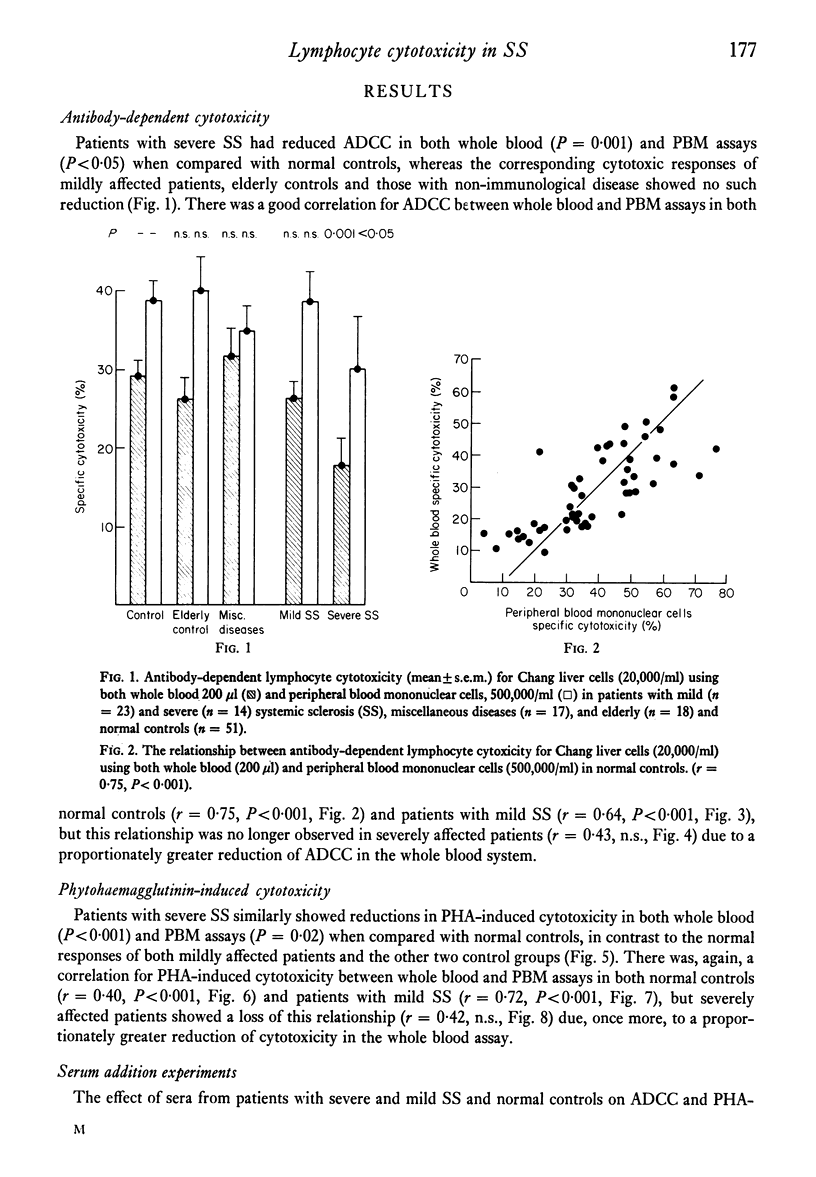
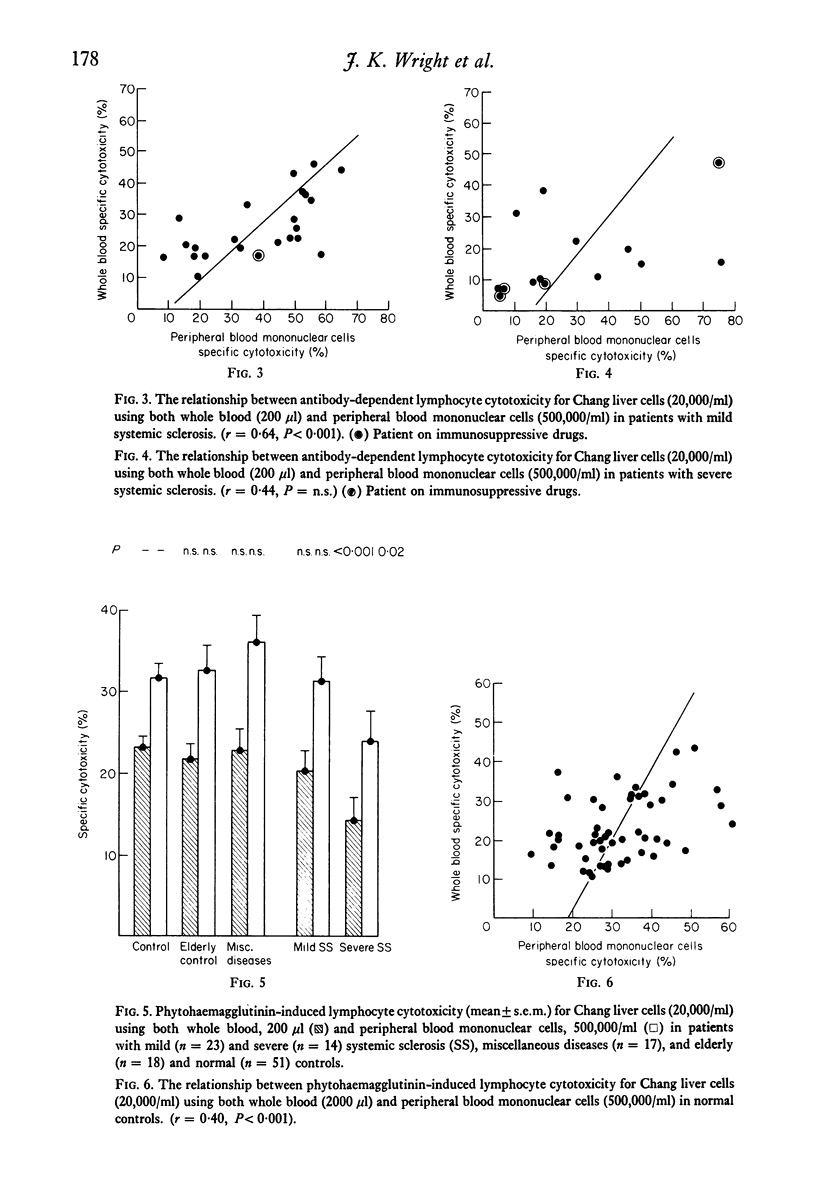
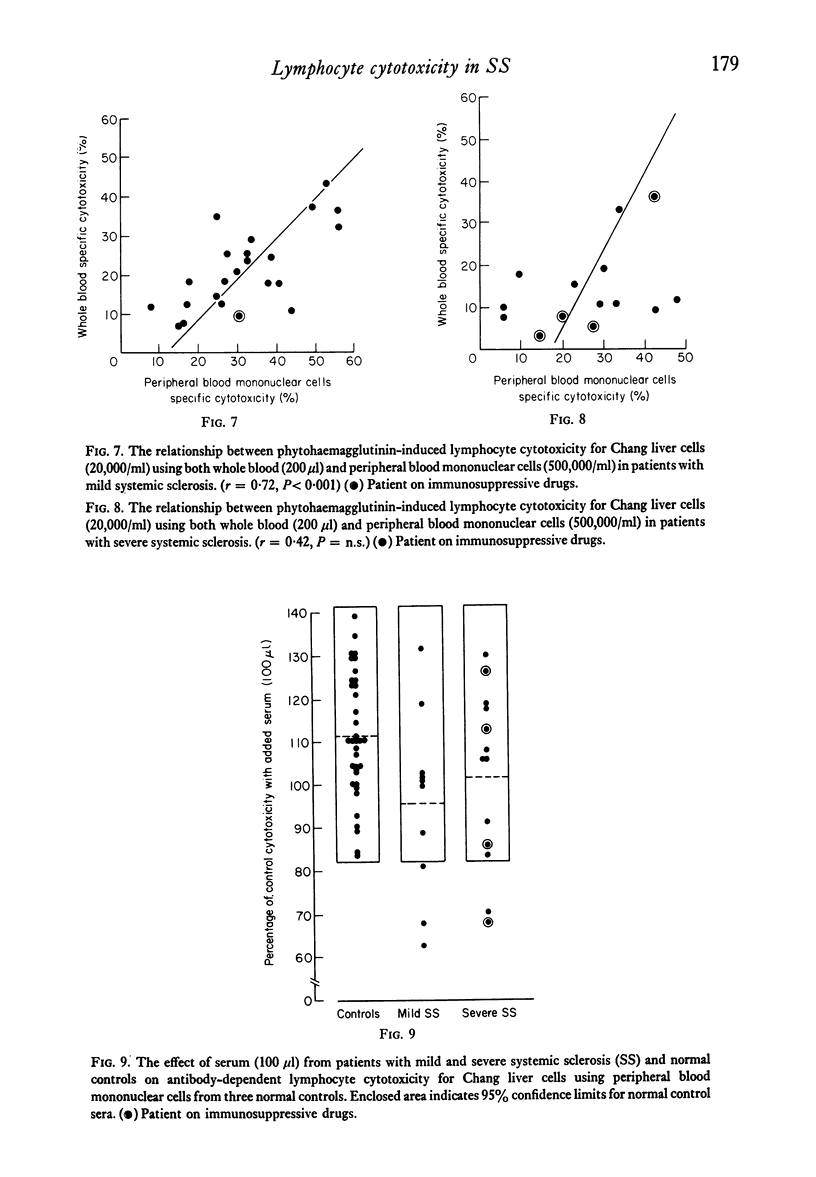
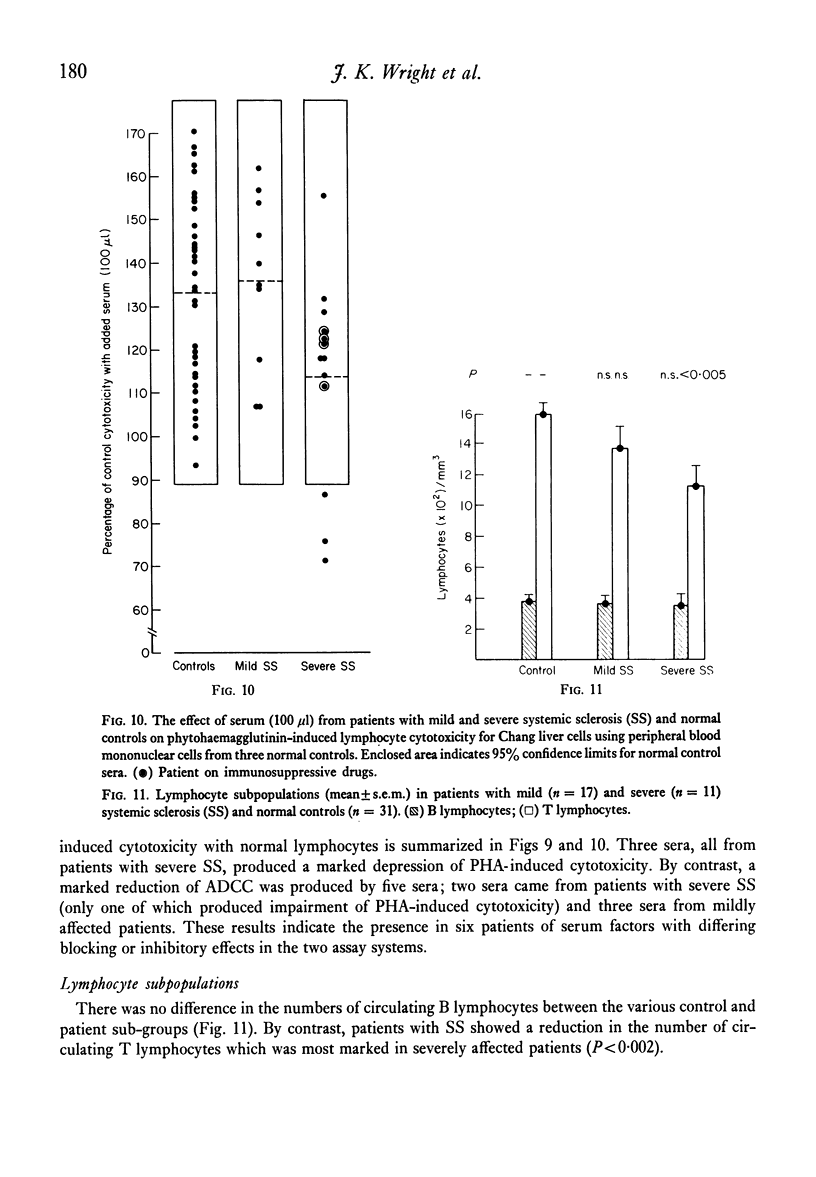
Cell-mediated cytotoxicity was examined in thirty-seven patients with systemic sclerosis using both whole blood and purified peripheral blood mononuclear cells (PBM) to measure antibody-dependent (ADCC) and phytohaemagglutinin (PHA) induced lymphocyte cytotoxicity to 51Cr-labelled Chang liver cells. In twenty-three mildly affected patients, ADCC and PHA-induced cytotoxicity did not differ from that found in control populations. By contrast, fourteen patients severely affected by extensive visceral disease showed reductions in both ADCC and PHA-induced cytotoxicity which were more marked in whole blood assays (P less than 0.001) than in those performed with PBM (P less than 0.05). The addition of patient's sera to control cytotoxicity assays suggested that blocking or suppressive serum factors could only account for some of the disproportionate reduction in whole blood cytotoxicity which, in the main, must be due to a lack of circulating effector cells. These results are in agreement with previous findings of reduced numbers of circulating thymus-dependent lymphocytes in patients with severe disease, a defect of cell-mediated immunity that may result from the chronic antigenic stimulation of an autoimmune disease process.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alarcón-Segovia D., Fishbein E., García-Ortigoza E., Estrada-Parra S. Uracil-specific anti-R.N.A. antibodies in scleroderma. Lancet. 1975 Feb 15;1(7903):363–366. doi: 10.1016/s0140-6736(75)91279-9. [DOI] [PubMed] [Google Scholar]
- BECK J. S., ANDERSON J. R., GRAY K. G., ROWELL N. R. ANTINUCLEAR AND PRECIPITATING AUTOANTIBODIES IN PROGRESSIVE SYSTEMIC SCLEROSIS. Lancet. 1963 Dec 7;2(7319):1188–1190. [PubMed] [Google Scholar]
- Cooperband S. R., Davis R. C., Schmid K., Mannick J. A. Competitive blockade of lymphocyte stimulation by a serum immuno-regulatory alpha globulin (IRA). Transplant Proc. 1969 Mar;1(1):516–523. [PubMed] [Google Scholar]
- Currie S., Saunders M., Knowles M. Immunological aspects of systemic sclerosis in vitro activity of lymphocytes from patients with the disorder. Br J Dermatol. 1971 May;84(5):400–409. doi: 10.1111/j.1365-2133.1971.tb02523.x. [DOI] [PubMed] [Google Scholar]
- Fenyk J. R., Jr, Smith C. M., Warkentin P. I., Krivit W., Goltz R. W., Neely J. E., Nesbit M. E., Ramsay N. K., Coccia P. F., Kersey J. H. Sclerodermatous graft-versus-host disease limited to an area of measles exanthem. Lancet. 1978 Mar 4;1(8062):472–473. doi: 10.1016/s0140-6736(78)90136-8. [DOI] [PubMed] [Google Scholar]
- Gratwohl A. A., Gratwhol A. A., Moutsopoulos H. M., Chused T. M., Akizuki M., Wolf R. O., Sweet J. B., Deisseroth A. B. Sjögren-type syndrome after allogeneic bone-marrow transplantation. Ann Intern Med. 1977 Dec;87(6):703–706. doi: 10.7326/0003-4819-87-6-703. [DOI] [PubMed] [Google Scholar]
- Hard R. C., Jr Hyperimmunoglobulinaemia, T-cell deficiency and plasmacytosis in RFM mice with host versus graft disease induced by the perinatal inoculations (T6XRFM)F1 spleen cells. Clin Exp Immunol. 1975 Nov;22(2):330–340. [PMC free article] [PubMed] [Google Scholar]
- Hersey P., Edwards A., Edwards J. Characterization of mononuclear effector cells in human blood. Clin Exp Immunol. 1976 Jan;23(1):104–113. [PMC free article] [PubMed] [Google Scholar]
- Hughes P., Holt S., Rowell N. R., Allonby I. D., Janis K., Dodd J. K. The relationship of defective cell-mediated immunity to visceral disease in systemic sclerosis. Clin Exp Immunol. 1977 May;28(2):233–240. [PMC free article] [PubMed] [Google Scholar]
- Hughes P., Holt S., Rowell N. R., Dodd J. Thymus-dependent (T) lymphocyte deficiency in progressive systemic sclerosis. Br J Dermatol. 1976 Nov;95(5):469–473. doi: 10.1111/j.1365-2133.1976.tb00855.x. [DOI] [PubMed] [Google Scholar]
- Johnson R. L., Ziff M. Lymphokine stimulation of collagen accumulation. J Clin Invest. 1976 Jul;58(1):240–252. doi: 10.1172/JCI108455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papamichail M., Brown J. C., Holborow E. J. Immunoglobulins on the surface of human lymphocytes. Lancet. 1971 Oct 16;2(7729):850–852. doi: 10.1016/s0140-6736(71)90224-8. [DOI] [PubMed] [Google Scholar]
- SHEARN M. A. Sjogren's syndrome in association with scleroderma. Ann Intern Med. 1960 Jun;52:1352–1362. doi: 10.7326/0003-4819-52-6-1352. [DOI] [PubMed] [Google Scholar]
- STASTNY P., STEMBRIDGE V. A., ZIFF M. HOMOLOGOUS DISEASE IN THE ADULT RAT, A MODEL FOR AUTOIMMUNE DISEASE. I. GENERAL FEATURES AND CUTANEOUS LESIONS. J Exp Med. 1963 Oct 1;118:635–648. doi: 10.1084/jem.118.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa M. D., Yang M., Lopes-Corrales E., Tan C., Hansen J. A., Dupont B., Good R. A. Ecotaxis: the principle and its application to the study of Hodgkin's disease. Clin Exp Immunol. 1977 Jan;27(1):143–151. [PMC free article] [PubMed] [Google Scholar]
- TUFFANELLI D. L. Scleroderma and its relationship to the "collagenoses": dermatomyositis, lupus erythematosus, rheumatoid arthritis and Sjogren's syndrome. Am J Med Sci. 1962 Feb;243:133–146. [PubMed] [Google Scholar]
- Terasaki P. I., Mottironi V. D., Barnett E. V. Cytotoxins in disease. Autocytotoxins in lupus. N Engl J Med. 1970 Oct 1;283(14):724–728. doi: 10.1056/NEJM197010012831403. [DOI] [PubMed] [Google Scholar]
- Van Vloten W. A., Scheffer E., Dooren L. J. Localized scleroderma-like lesions after bone marrow transplantation in man. A chronic graft versus host reaction. Br J Dermatol. 1977 Apr;96(4):337–341. doi: 10.1111/j.1365-2133.1977.tb07126.x. [DOI] [PubMed] [Google Scholar]
- Wisloff F., Froland S. S., Michaelsen T. E. Characterization of subpopulations of human lymphoid cells participating in phytohemagglutinin and concanavalin A-induced cytotoxicity. Int Arch Allergy Appl Immunol. 1974;47(4):488–497. doi: 10.1159/000231243. [DOI] [PubMed] [Google Scholar]


