FIG. 1.
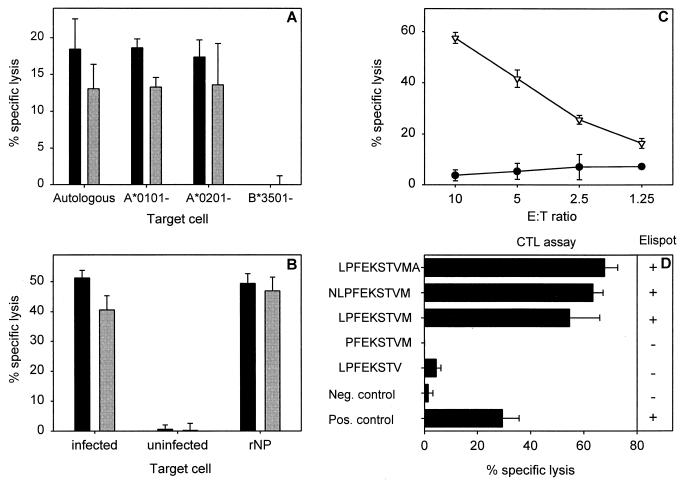
Characterization of an HLA-B*3501-restricted epitope and CTL clone (1980-1). (A) HLA class I restriction of CTL clone 1980-1 was determined by partially mismatched target cells (BLCL) infected with influenza A (H3N2) virus (Resvir-9) in a 51Cr release assay at effector-to-target cell (E:T) ratios of 10:1 (black bars) and 5:1 (grey bars) as described previously (3). Autologous BLCL cells (HLA-A*0101, HLA-A*0201, HLA-B*0801, and HLA-B*3501) and BLCL cells mismatched for a single HLA class I molecule (HLA-A*0101−, HLA-A*0201−, and HLA-B*3501−) were used. All BLCL cells expressed HLA-B*0801. The percent lysis of uninfected target cells was subtracted from the percent lysis of target cells infected with influenza virus. (B) Protein specificity of an HLA-B*3501-restricted CTL clone. HLA-B*3501+ target cells were infected with influenza A (H3N2) virus (Resvir-9) or were left uninfected and used as positive and negative controls, respectively. In addition, HLA-B*3501+ target cells were incubated overnight with 100 μg of bacterially expressed rNP of A/Netherlands/18/94 (A/H3N2)/ml as previously described (33). The target cells were used in a 51Cr release assay with CTL clone 1980-1 at E:T ratios of 10:1 (black bars) and 5:1 (grey bars). (C) A 9-mer peptide (NP418-426; LPFEKSTVM) (▿), predicted with an HLA binding prediction program (25), was loaded onto HLA-B*3501+ BLCL cells and used as target cells in a 51Cr release assay with the T-cell clone 1980-1 at the indicated E:T ratios. Untreated BLCL cells (•) were included as a negative control. (D) Minimal epitope mapping of HLA-B*3501-restricted epitope. Peptides were synthesized based on the initial 9-amino-acid sequence LPFEKSTVM that was extended or truncated at the C- or N-terminal end of the NP418-426 epitope in order to determine the minimal epitope. BLCL cells loaded with 5 μM concentrations of the different peptides were used as target cells in a CTL assay at an E:T ratio of 5:1 or applied to stimulate the CTL clone (1980-1) in an enzyme-linked immunospot assay. A plus indicates gamma interferon (IFN-γ) production by the CTL clone, while a minus indicates no production of IFN-γ. Influenza virus (Resvir-9)-infected BLCL cells and uninfected BLCL cells were included in both assays as positive and negative controls, respectively. Percent lysis is given as the mean ± standard deviation, and the results are representative of multiple experiments.