Abstract
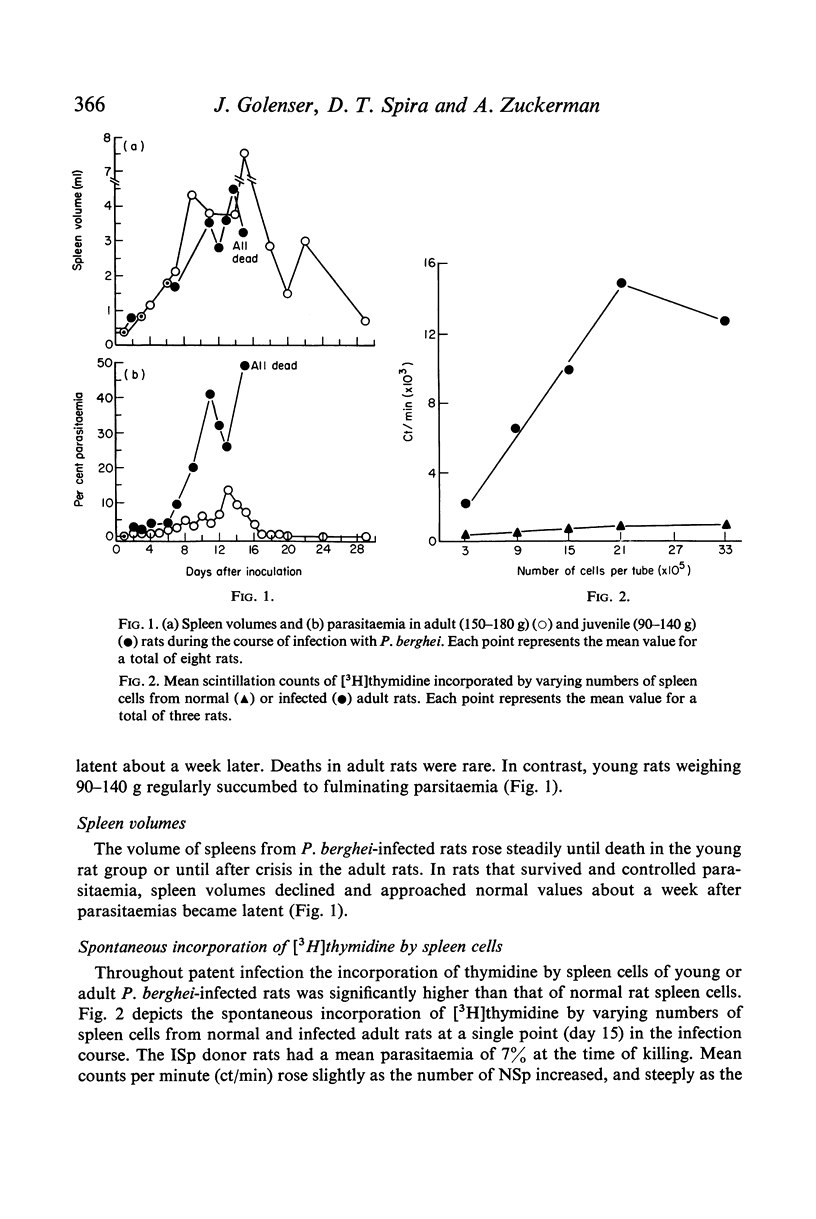
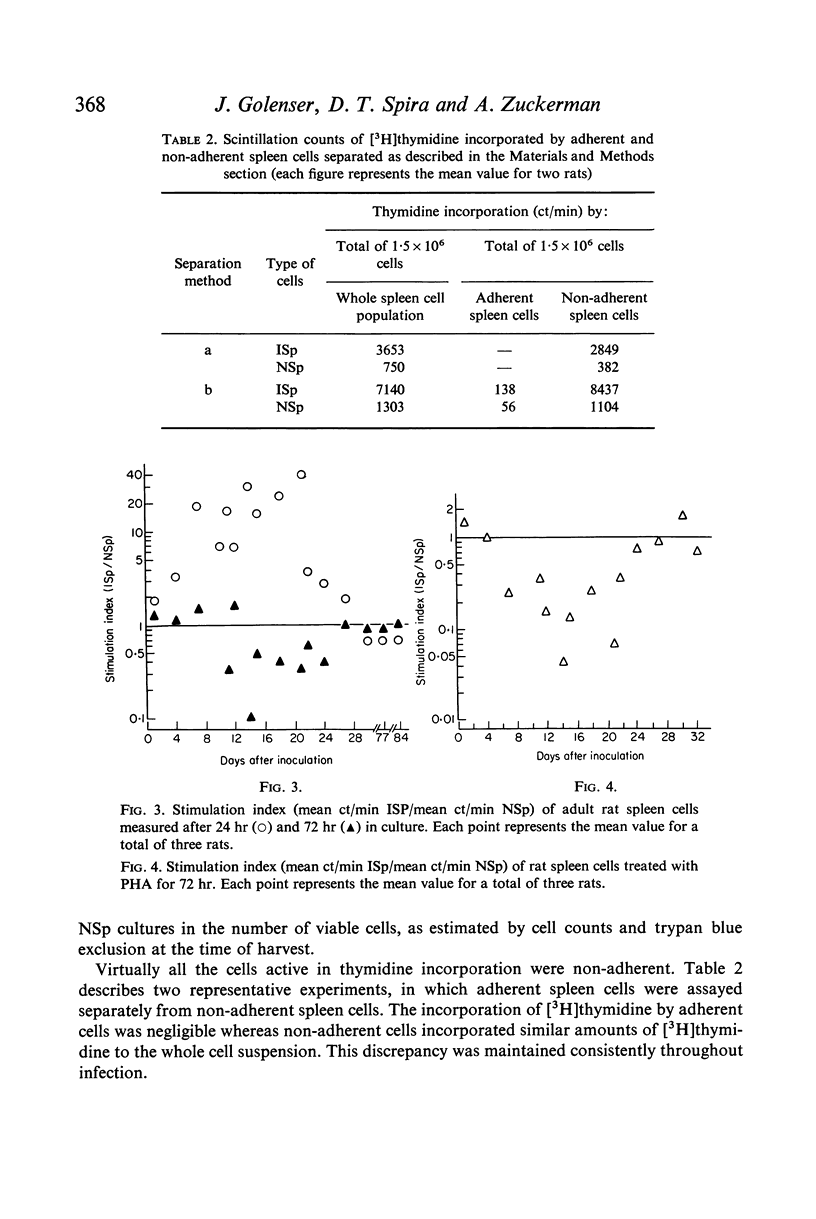
The in vitro incorporation of thymidine by spleen cells of rat infected with Plasmodium berghei was higher than that of normal rat spleen cells. The incorporation was proportional to the number of cells in the system and was related to the day of infection, e.g. spleen cells from malarious rats incorporated up to forty times as much thymidine as did normal spleen cells 21 days after inoculation. At the same time, the response of the malarious spleen cells to phytohaemagglutinin (PHA) was only one-twentieth of that observed for normal spleen cells. The thymidine was incorporated by non-adherent spleen cells in a single pulse during the first 8 hr in culture.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAXTER C. F., BELCHER E. H., HARRISS E. B., LAMERTON L. F. Anaemia and erythropoiesis in the irradiated rat: an experimental study with particular reference to techniques involving radioactive iron. Br J Haematol. 1955 Jan;1(1):86–103. doi: 10.1111/j.1365-2141.1955.tb05491.x. [DOI] [PubMed] [Google Scholar]
- CURTAIN C. C., KIDSON C., CHAMPNESS D. L., GORMAN J. G. MALARIA ANTIBODY CONTENT OF GAMMA 2-7S GLOBULIN IN TROPICAL POPULATIONS. Nature. 1964 Sep 26;203:1366–1367. doi: 10.1038/2031366a0. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Gery I., Waksman B. H. Suppressive effects of in vivo immunization on PHA responses in vitro. J Immunol. 1974 Jan;112(1):215–221. [PubMed] [Google Scholar]
- Greenwood B. M., Playfair J. H., Torrigiani G. Immunosuppression in murine malaria. I. General characteristics. Clin Exp Immunol. 1971 Mar;8(3):467–478. [PMC free article] [PubMed] [Google Scholar]
- Greenwood B. M. Possible role of a B-cell mitogen in hypergammaglobulinaemia in malaria and trypanosomiasis. Lancet. 1974 Mar 16;1(7855):435–436. doi: 10.1016/s0140-6736(74)92386-1. [DOI] [PubMed] [Google Scholar]
- Mitchell M. S., Kirkpatrick D., Mokyr M. B., Gery I. On the mode of action of BCG. Nat New Biol. 1973 Jun 13;243(128):216–218. doi: 10.1038/newbio243216a0. [DOI] [PubMed] [Google Scholar]
- Moore D. L., Heyworth B., Brown J. PHA-induced lymphocyte transformations in leucocyte cultures from malarious, malnourished and control Gambian children. Clin Exp Immunol. 1974 Aug;17(4):647–656. [PMC free article] [PubMed] [Google Scholar]
- Osunkoya B. O., Williams A. I., Reddy S. Spontaneous lymphocyte transformation in leucocyte cultures of children with falciparum malaria. Trop Geogr Med. 1972 Jun;24(2):157–161. [PubMed] [Google Scholar]
- Phillips R. S. Plasmodium berghei: passive transfer of immunity by antisera and cells. Exp Parasitol. 1970 Jun;27(3):479–495. doi: 10.1016/0014-4894(70)90052-4. [DOI] [PubMed] [Google Scholar]
- SINGER I. The cellular reactions to infections with Plasmodium berghei in the white mouse. J Infect Dis. 1954 May-Jun;94(3):241–261. doi: 10.1093/infdis/94.3.241. [DOI] [PubMed] [Google Scholar]
- Scott M. T. Biological effects of the adjuvant Corynebacterium parvum. I. Inhibition of PHA, mixed lymphocyte and GVH reactivity. Cell Immunol. 1972 Nov;5(3):459–468. doi: 10.1016/0008-8749(72)90072-x. [DOI] [PubMed] [Google Scholar]
- ZUCKERMAN A. Blood loss and replacement in plasmodial infections. I. Plasmodium berghei in untreated rats of varying age and in adult rats with erythropoietic mechanisms manipulated before inoculation. J Infect Dis. 1957 Mar-Apr;100(2):172–206. doi: 10.1093/infdis/100.2.172. [DOI] [PubMed] [Google Scholar]


