Abstract
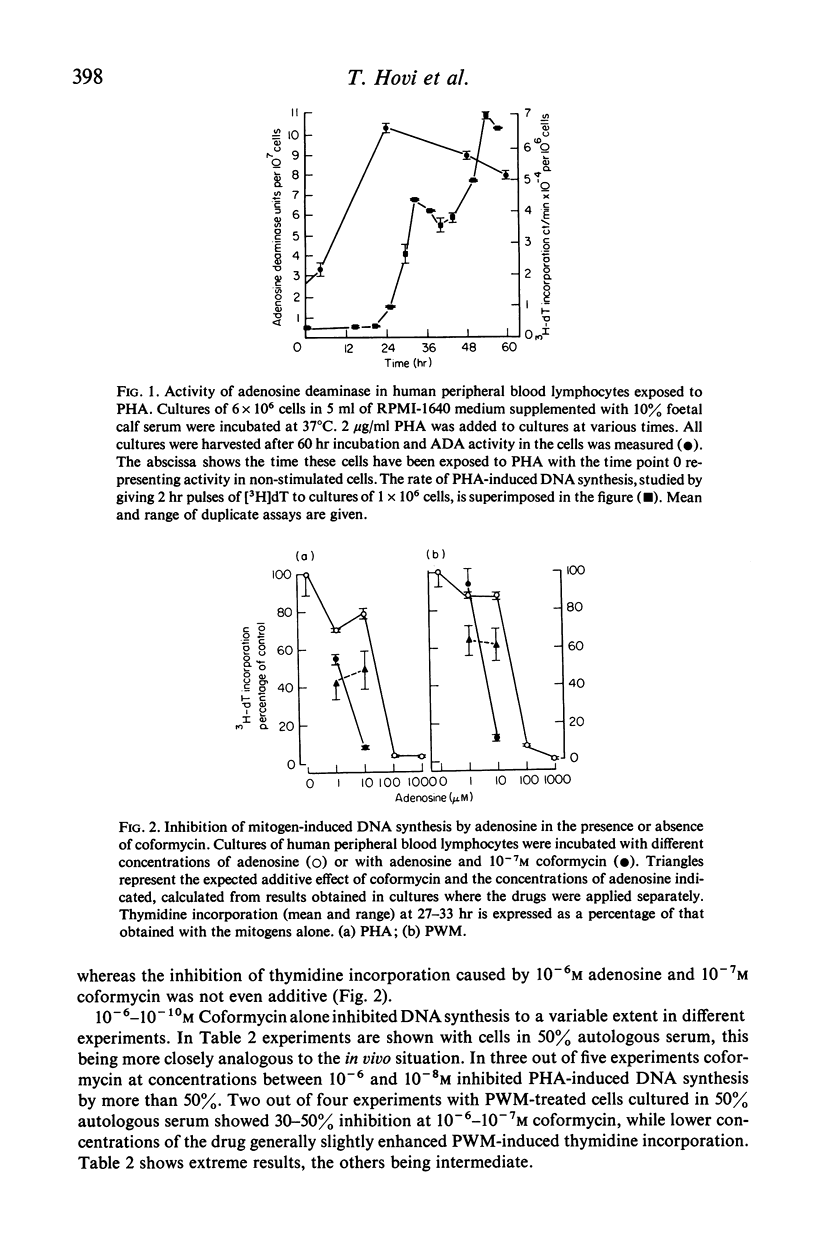
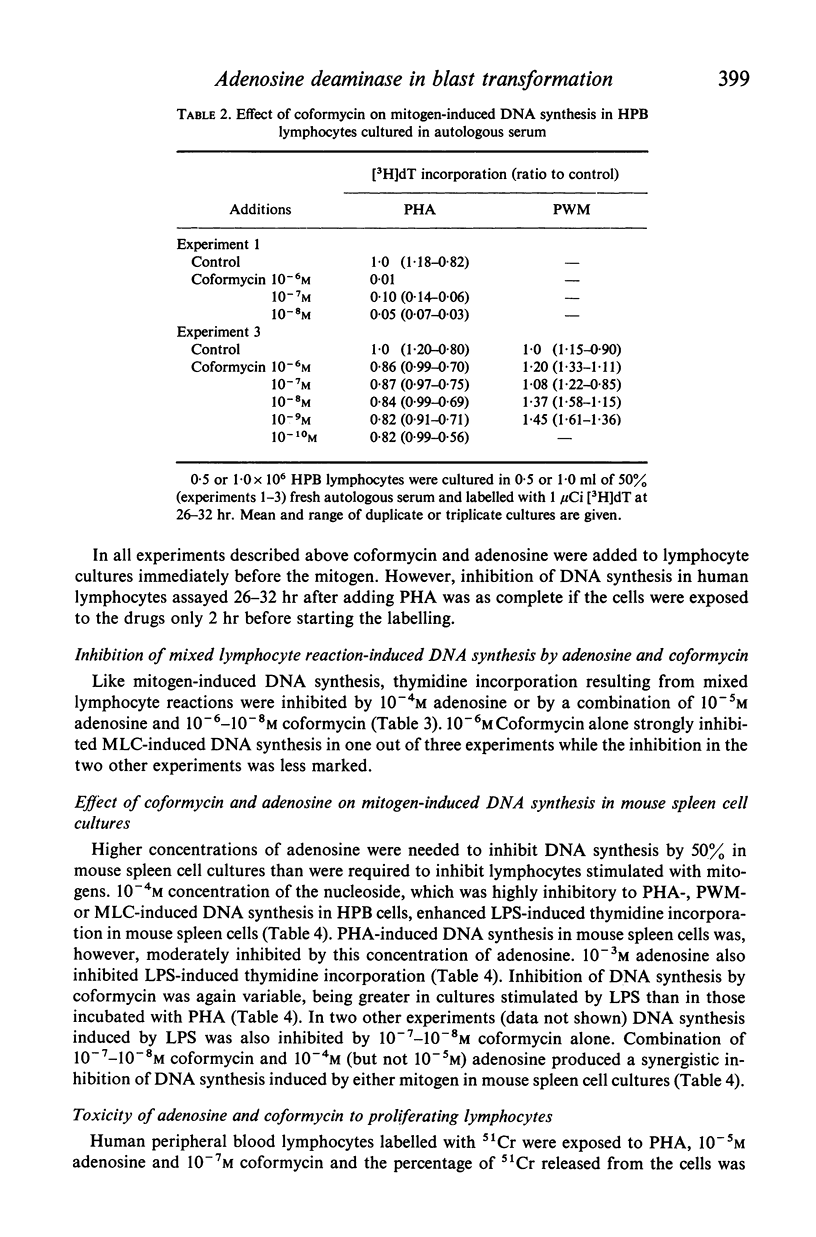
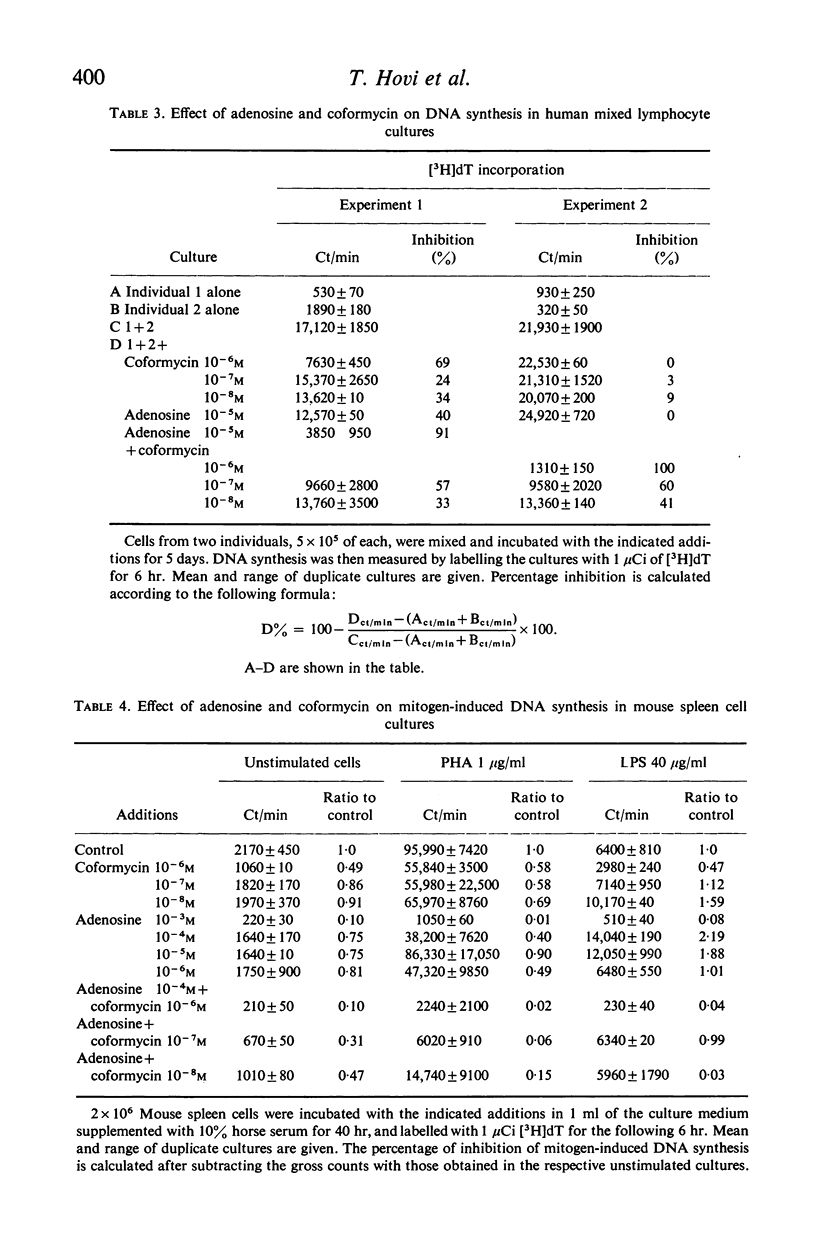
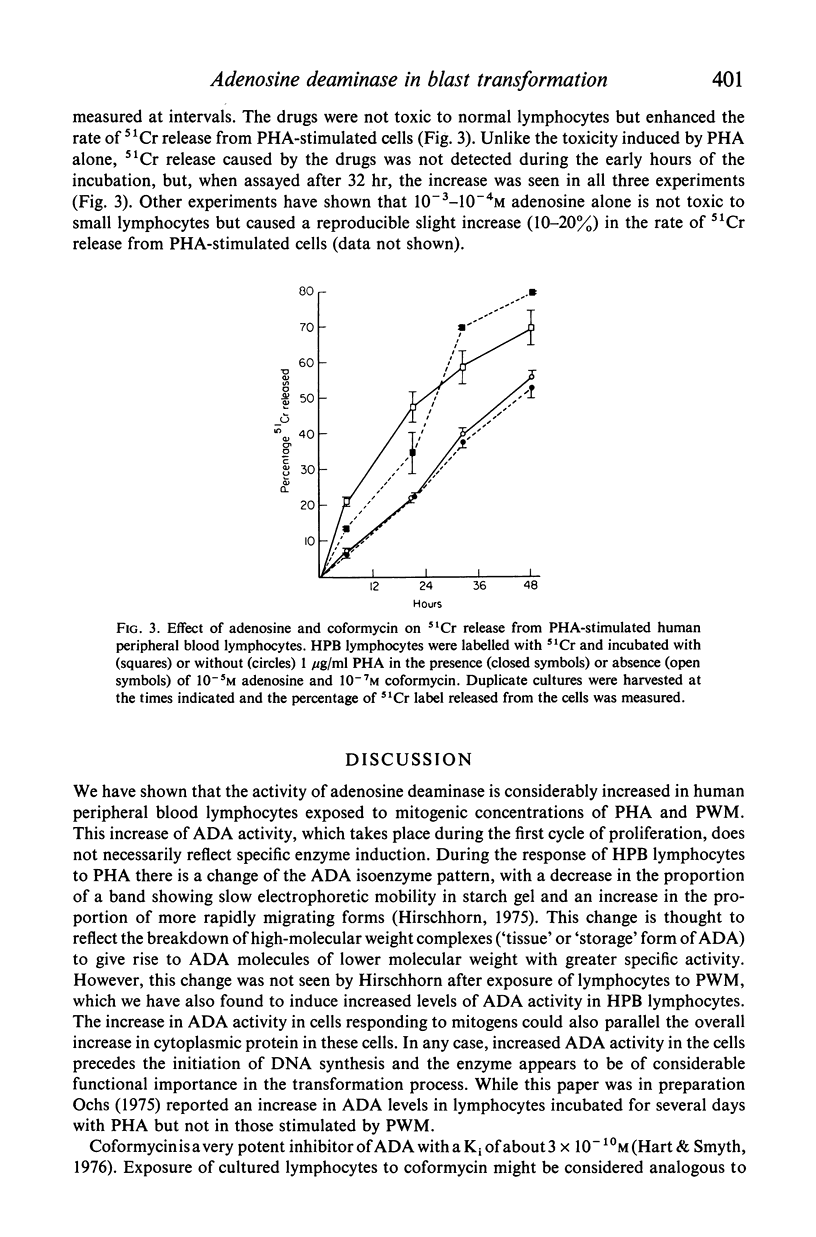
Activity of adenosine deaminase (ADA), an enzyme known to be deficient in some patients with severe combined immunodeficiency, increased three-fold within a 24-hour exposure of human peripheral blood lymphocytes to phytohaemagglutinin (PHA) in culture. This increase took place before the onset of DNA synthesis. Increased levels of ADA activity were also observed in lymphocytes incubated with pokeweed mitogen (PWM) for 60 hr. DNA synthesis induced by PHA, PWM or mixed lymphocyte cultures (MLC) was strongly inhibited by adenosine at concentrations of 10(-4) M or higher when human peripheral blood lymphocytes were cultured in a medium supplemented with horse serum, which lacks ADA. 10(-6)-10(-8) M coformycin, a potent inhibitor of ADA, inhibited PHA-, PWM- and MLC-induced DNA synthesis to a variable extent, whereas thymidine incorporation induced by Salmonella lipopolysaccharide (LPS) in mouse spleen cell cultures was strongly inhibited (by 75% or more) by 10(-6) M coformycin. Combination of 10(-7)-10(-8) M coformycin and 10(-4)-10(-5) M adenosine synergistically inhibited mitogen- or MLC-induced DNA synthesis in human and mouse lymphocyte cultures. These results, together with observations on children with ADA deficiency, provide evidence that adenosine deaminase is highly important for lymphocyte proliferation. Human peripheral blood lymphocytes incubated with PHA, 10(-5) M adenosine and 10(-7) M coformycin showed some cytotoxicity whereas the rate of 51Cr release from normal lymphocytes was not modified by the drugs. These findings suggest that in vivo clones of lymphocytes responding to specific antigens might be eliminated by coformycin, which may prove to be useful as a specific immunosuppressive agent.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADY T. G., O'DONOVAN C. I. A STUDY OF THE TISSUE DISTRIBUTION OF ADENOSINE DEAMINASE IN SIX MAMMAL SPECIES. Comp Biochem Physiol. 1965 Jan;14:101–120. doi: 10.1016/0010-406x(65)90011-3. [DOI] [PubMed] [Google Scholar]
- Brady T. Adenosine deaminase. Biochem J. 1942 Jun;36(5-6):478–484. doi: 10.1042/bj0360478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway E. J., Cooke R. The deaminases of adenosine and adenylic acid in blood and tissues. Biochem J. 1939 Apr;33(4):479–492. doi: 10.1042/bj0330479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissing J., Knudsen B. Adenosine-deaminase deficiency and combined immunodeficiency syndrome. Lancet. 1972 Dec 16;2(7790):1316–1316. doi: 10.1016/s0140-6736(72)92692-x. [DOI] [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. Human phosphoribosylpyrophosphate synthetase. Kinetic mechanism and end product inhibition. J Biol Chem. 1972 Apr 10;247(7):2126–2131. [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. Phosphoribosylpyrophosphate in man: biochemical and clinical significance. Ann Intern Med. 1971 Mar;74(3):424–433. doi: 10.7326/0003-4819-74-3-424. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Green H., Chan T. Pyrimidine starvation induced by adenosine in fibroblasts and lymphoid cells: role of adenosine deaminase. Science. 1973 Nov 23;182(4114):836–837. doi: 10.1126/science.182.4114.836. [DOI] [PubMed] [Google Scholar]
- HALL J. G. Adenosine deaminase activity in lymphoid cells during antibody production. Aust J Exp Biol Med Sci. 1963 Feb;41:93–97. doi: 10.1038/icb.1963.8. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Grossman J., Weissmann G. Effect of cyclic 3',5'-adenosine monophosphate and theophylline on lymphocyte transformation. Proc Soc Exp Biol Med. 1970 Apr;133(4):1361–1365. doi: 10.3181/00379727-133-34690. [DOI] [PubMed] [Google Scholar]
- Hovi T., Allison A. C., Allsop J. Rapid increase of phosphoribosyl pyrophosphate concentration after mitogenic stimulation of lymphocytes. FEBS Lett. 1975 Jul 15;55(1):291–293. doi: 10.1016/0014-5793(75)81014-3. [DOI] [PubMed] [Google Scholar]
- Ishii K., Green H. Lethality of adenosine for cultured mammalian cells by interference with pyrimidine biosynthesis. J Cell Sci. 1973 Sep;13(2):429–439. doi: 10.1242/jcs.13.2.429. [DOI] [PubMed] [Google Scholar]
- Seegmiller J. E., Rosenbloom F. M., Kelley W. N. Enzyme defect associated with a sex-linked human neurological disorder and excessive purine synthesis. Science. 1967 Mar 31;155(3770):1682–1684. doi: 10.1126/science.155.3770.1682. [DOI] [PubMed] [Google Scholar]
- Smyth J. F., Harrap K. R. Adenosine deaminase activity in leukaemia. Br J Cancer. 1975 May;31(5):544–549. doi: 10.1038/bjc.1975.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolberg G., Zimmerman T. P., Hiemstra K., Winston M., Chu L. C. Adenosine inhibition of lymphocyte-mediated cytolysis: possible role of cyclic adenosine monophosphate. Science. 1975 Mar 14;187(4180):957–959. doi: 10.1126/science.167434. [DOI] [PubMed] [Google Scholar]
- Wong P. C., Murray A. W. 5-Phosphoribosyl pyrophosphate synthetase from Ehrlich ascites tumor cells. Biochemistry. 1969 Apr;8(4):1608–1614. doi: 10.1021/bi00832a042. [DOI] [PubMed] [Google Scholar]


