Figure 3.
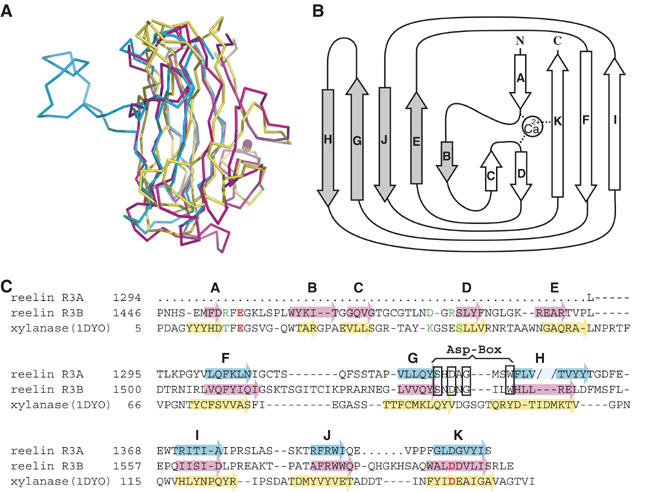
Subrepeat structure. (A) Superposition of subrepeat A (cyan), subrepeat B (magenta), and a CBM from bacterial xylanase (1DYO, yellow). (B) Topology diagram for the secondary structure of subrepeat B. Five strands (gray arrows) form one β-sheet on the concave side, whereas the remaining strands (open arrows) form the other sheet on the convex side. (C) Structural alignment of two reelin subrepeats and CBM of xylanase. β-Strands are indicated by arrows color-coded as in (A). The break created by the loop insertion at strand H in subrepeat A is indicated by the pale color. Ca2+-coordinating residues in subrepeat B and 1DYO are colored in red (side-chain coordination) or green (main-chain coordination). ‘Asp-box' signature motif is boxed.
