Figure 4.
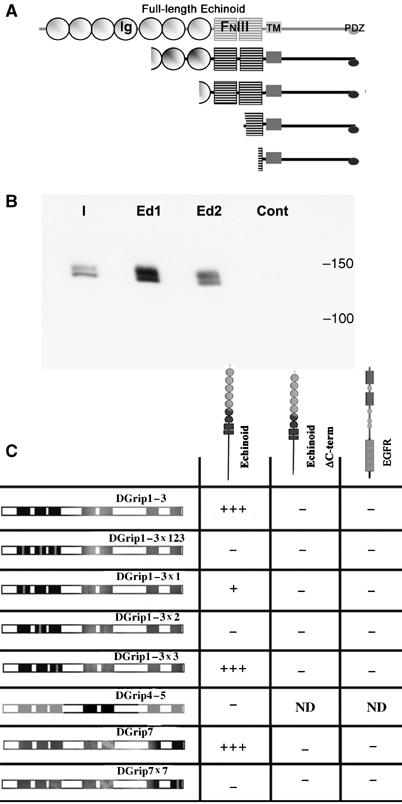
The cell-adhesion molecule Echinoid physically interacts with Dgrip. (A) Y2H screen performed using the first three PDZDs of DGrip as bait returned four independent isolates of the Immunoglobulin (Ig) and Fibronectin type III (FNIII)-domain containing cell adhesion molecule Echinoid (Ed). All isolates contained the transmembrane (TM) region and the entire cytosolic tail, including the EIIV PDZ-ligand motif. (B) Full-length, C-terminally myc-tagged DGrip expressed in Sf.9 cells specifically binds to a 10aa peptide representing the C-terminus of Ed. Shown is the input (I), binding of DGrip-myc to the Ed peptide (Ed1/2, two independent experiments), and binding to a 10aa scrambled control peptide (cont). (C) Y2H experiments reveal a specific pattern of Ed binding to DGrip PDZDs. The Ed cytosolic tail strongly interacts with a construct containing the first three PDZDs of DGrip, or containing PDZD 7 only (+++). This interaction was abolished by point mutation of PDZDs 2 or 7 (−), strongly reduced by point mutation of PDZD 1 (+) and unaffected by point mutation of PDZD 3 (+++). This interaction depended on the EIIV motif at the C-terminal of Ed, as Ed-ΔC-term did not interact with DGrip constructs. DGrip did not interact with the EGFR C-terminus, used here as a control, which has a C-terminal type I PDZ-ligand motif (ETRV).
