Abstract
In an in vitro assessment of antimalarial combinations, dihydroartemisinin (DHA) showed no interaction or was mildly antagonistic when combined with piperaquine, pyronaridine, or naphthoquine. Interactions between 4-aminoquinolines and related drugs were also indifferent/antagonistic. The clinical significance of mildly antagonistic DHA combinations is uncertain but may become important if parasite drug sensitivity declines.
A number of studies have investigated the in vitro sensitivity of Plasmodium falciparum to the bisquinoline piperaquine (8), but its interactions with other antimalarial agents have not been assessed. Such interaction studies are important because piperaquine is increasingly used as the long-acting component of artemisinin combination therapy (ACT) with dihydroartemisinin (DHA) (9). Although clinical cure rates for piperaquine-DHA are high (10, 23), there may be situations, such as when treatment is incomplete, in which an antagonistic interaction could become significant. In addition, piperaquine-DHA might be given when chemoprophylaxis or treatment including long-half-life drugs such as chloroquine or mefloquine has failed. Antagonism between piperaquine and such prior therapy could increase the risk of late treatment failure. Our aim was, therefore, to investigate in vitro interactions between piperaquine and both DHA and conventional antimalarials. Because pyronaridine and naphthoquine are promising components of ACT (9), we also assessed their interaction profiles.
Drug interactions were assessed using the laboratory-adapted P. falciparum clones 3D7 (chloroquine sensitive) and K1 (chloroquine resistant) and using isobolographic analysis (6, 19). DHA was obtained from Dafra Pharma NV (Turnhout, Belgium), piperaquine tetraphosphate from Yick-Vic Chemicals and Pharmaceuticals Ltd. (Kowloon, Hong Kong), chloroquine diphosphate and quinine hydrochloride from Sigma Chemical Co. (St Louis, MO), mefloquine hydrochloride from Roche Products Pty. Ltd. (Dee Why, Australia), naphthoquine phosphate from ZYF Pharm Chemical (Shanghai, China), and pyronaridine from the Chinese Academy of Preventative Medicine (Shanghai, China).
Cultures were maintained in modified candle jars (22), and drug activity was determined from 3H-hypoxanthine incorporation (5, 12). Drug dilutions (1 × 10−4 to 5 × 10−10 M) were aliquoted into triplicate wells of sterile 96-well microtiter plates. Parasite cultures (2% parasitemia, 1% hematocrit) and 0.5 μCi 3H-hypoxanthine were added, and the mixture was incubated for 48 h, harvested, and counted. The concentrations of individual drugs required to inhibit parasite growth by 50% (IC50) were determined by linear interpolation (15). Fractional inhibitory concentrations (FICs) of each agent were then titrated against those of each of the other agents in further 48-hour inhibition assays. All experiments involving piperaquine were repeated, but other interactions were assessed from a single triplicate experiment. Experiments with K1 examined only interactions with piperaquine.
Concentrations of each drug that alone or in combination resulted in 50% growth inhibition were plotted as FICs (1). We used two approaches to analyze these isoboles (14). First, the function Yi = 1 − {XI/[XI + e(−I)(1 − Xi)] } (4) was fitted to the data (SAAM II; SAAM Institute, Seattle, WA), where Yi is the IC50 of drug A combined with drug B, Xi is the IC50 of drug B when combined with drug A, and I is the interaction value. Positive I values indicate synergy, negative values indicate antagonism, and values close to zero represent no interaction. Second, sums (Σ) of FICs were calculated from the following formula: (IC50 of A in a mixture resulting in 50% inhibition/IC50 of A alone) + (IC50 of B in a mixture resulting in a 50% inhibition/IC50 of B alone) (1). A ΣFIC of <1 indicates synergy, >1 indicates antagonism, and close to 1 indicates no interaction. Values of I and ΣFIC were considered significantly different from no interaction if both 95% confidence intervals (CIs) of the estimates did not span zero and unity, respectively.
The results of isobolographic analysis are shown in Table 1. No synergistic combinations were identified. For piperaquine-DHA, there was no interaction present for 3D7 and there was antagonism for K1. In the case of pyronaridine and naphthoquine, there was antagonism between these two drugs and DHA. The remaining combinations showed no interaction or were antagonistic. Based on the values and 95% CIs of I and ΣFIC for both clones, the strongest antagonism was between piperaquine and mefloquine (Fig. 1).
TABLE 1.
In vitro efficacy of antimalarial drug combinations against P. falciparum clones 3D7 and K1 as assessed by isobolographic analysisc
| Drug combination | Results with clone:
|
|||||
|---|---|---|---|---|---|---|
| 3D7
|
K1
|
|||||
| I (95% CI) | ΣFIC (95% CI) | Interpretation | I (95% CI) | ΣFIC (95% CI) | Interpretation | |
| Piperaquine plus | ||||||
| DHA | −0.30 (−0.82 to 0.23) | 0.99 (0.91 to 1.07) | No interaction | −1.98a (−3.39 to −0.57) | 1.39b (1.24 to 1.55) | Antagonism |
| Quinine | −2.11a (−3.41 to −0.80) | 1.19b (1.03 to 1.36) | Antagonism | 0.04 (−4.21 to 4.30) | 1.26 (0.89 to 1.64) | No interaction |
| Mefloquine | −3.44a (−4.39 to −2.49) | 1.31b (1.16 to 1.46) | Antagonism | −1.30a (−2.25 to −0.36) | 1.28b (1.07 to 1.49) | Antagonism |
| Chloroquine | 0.42 (−0.33 to 1.17) | 0.94 (0.79 to 1.09) | No interaction | −1.57a (−2.03 to −1.11) | 1.11 (0.81 to 1.42) | No interaction |
| Pyronaridine | −0.88a (−1.33 to −0.44) | 1.05 (0.98 to 1.13) | No interaction | 0.18 (−0.33 to 0.68) | 0.99 (0.89 to 1.08) | No interaction |
| Naphthoquine | −1.88a (−2.73 to −1.04) | 1.15 (0.87 to 1.44) | No interaction | −0.50a (−0.97 to −0.02) | 1.12b (1.06 to 1.18) | Antagonism |
| Pyronaridine plus | ||||||
| DHA | −2.68a (−3.24 to −2.12) | 1.32b (1.17 to 1.48) | Antagonism | |||
| Quinine | −1.29 (−2.60 to 0.03) | 1.22b (1.02 to 1.43) | No interaction | |||
| Mefloquine | −2.45a (−2.86 to −2.05) | 1.32b (1.12 to 1.51) | Antagonism | |||
| Chloroquine | 0.01 (−1.63 to 1.66) | 0.90 (0.64 to 1.16) | No interaction | |||
| Naphthoquine plus | ||||||
| DHA | −1.50a (−2.03 to −0.97) | 1.22b (1.12 to 1.33) | Antagonism | |||
| Quinine | −0.03 (−1.18 to 1.12) | 1.02 (0.82 to 1.23) | No interaction | |||
| Mefloquine | 0.22 (−0.72 to 1.16) | 1.02 (0.82 to 1.23) | No interaction | |||
| Chloroquine | −2.40a (−3.63 to −1.18) | 1.26b (1.12 to 1.39) | Antagonism | |||
Significantly different from zero (P < 0.05).
Significantly different from 1 (P < 0.05).
Data are the interaction factor (I) and the summed fractional inhibitory concentration (ΣFIC) with 95% CIs. Each interpretation is based on both I and ΣFIC results for each combination and clone.
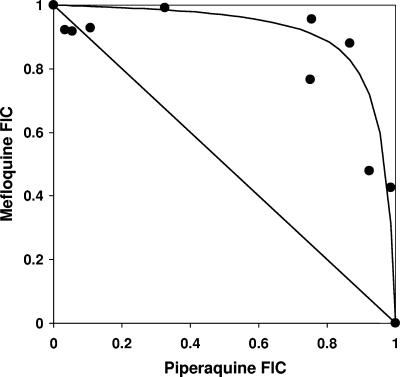
FIG. 1.
Isobologram describing the antagonistic interaction between piperaquine and mefloquine on culture-adapted clone 3D7. The FICs of the drugs that alone or in combination induced 50% growth inhibition are plotted. The line of additivity (joining FICs of 1.0) is shown.
All combinations tested against the two laboratory-adapted clones had ΣFIC values of >0.5 and ≤4.0, the range regarded as showing an indifferent interaction between two antimicrobial agents (17). This suggests that the rapid parasiticidal effect of DHA in vivo (11) would be preserved when given with piperaquine and that there are similarly no concerns with the other combinations assessed. There are, however, alternative analyses and interpretations of the data, especially since even minor drug interactions can be clinically significant (2). In addition, since the reproducibility of a single analysis method is one reason for the choice of a conservative ΣFIC range (17), the two independent methods we used should increase confidence in our interaction measures.
Most experiments involving DHA showed I and ΣFIC values suggestive of mild antagonism. In the case of pyronaridine, the absolute value of I (2.68) was within the range (2.43 to 2.88) used to identify atovaquone-proguanil as a suitable synergistic combination for clinical development (4). Antagonism between artemisinin derivatives and quinolines and related drugs has been reported previously (4, 18), but other authors have found synergy (13) and there is evidence of strain-specific differences in interactions between conventional antimalarial agents (13) such as in the present study. Moreover, piperaquine, pyronaridine, and naphthoquine have been incorporated successfully as part of ACT in field studies (9, 10, 23, 24), suggesting that a mild antagonistic interaction is unlikely to be of any short-term clinical significance. It is, nevertheless, possible that this situation will change as parasite sensitivity to artemisinin derivatives wanes (16).
Previous studies have shown that quinoline drug combinations such as chloroquine-quinine (20, 21) are antagonistic. Our results are consistent with these data but, if present, antagonism was mild. The most marked, that between mefloquine and piperaquine, may, however, have clinical implications. Mefloquine is given commonly as a component of ACT and, in part because of its 2-week half-life, has a role as single-drug chemoprophylaxis (7). If piperaquine-DHA treatment is given when mefloquine has been taken recently, initial parasite clearance may be rapid because mefloquine and the short-half-life artemisinin derivatives have synergistic effects on P. falciparum (3). However, late recrudescences may be more likely when only relatively low, antagonistic plasma concentrations of mefloquine and piperaquine remain.
In summary, the potential in vivo significance of mildly antagonistic in vitro interactions between DHA and quinoline/bisquinoline/aryl aminoalcohol drugs, and among most long-half-life quinoline and related antimalarials, is unknown but is likely to be minimal. Nevertheless, such interactions may have clinical consequences in particular circumstances, and their importance could increase with changes in parasite drug sensitivity.
Acknowledgments
This study was supported by the National Health and Medical Research Council (NHMRC) of Australia (grant 353663). P.H.R.B. is a Senior Research Fellow of the NHMRC.
REFERENCES
- 1.Berenbaum, M. C. 1978. A method for testing for synergy with any number of agents. J. Infect. Dis. 137:122-130. [DOI] [PubMed] [Google Scholar]
- 2.Berenbaum, M. C. 1987. Minor synergy and antagonism may be clinically important. J. Antimicrob. Chemother. 19:271-273. [DOI] [PubMed] [Google Scholar]
- 3.Bwijo, B., M. H. Alin, N. Abbas, W. Wernsdorfer, and A. Bjorkman. 1997. Efficacy of artemisinin and mefloquine combinations against Plasmodium falciparum. In vitro simulation of in vivo pharmacokinetics. Trop. Med. Int. Health 2:461-467. [PubMed] [Google Scholar]
- 4.Canfield, C. J., M. Pudney, and W. E. Gutteridge. 1995. Interactions of atovaquone with other antimalarial drugs against P. falciparum in vitro. Exp. Parasitol. 80:373-381. [DOI] [PubMed] [Google Scholar]
- 5.Chulay, J. D., J. D. Haynes, and C. L. Diggs. 1983. P. falciparum: assessment of in vitro growth by [3H]-hypoxanthine incorporation. Exp. Parasitol. 55:138-146. [DOI] [PubMed] [Google Scholar]
- 6.Czarniecki, C. W., C. W. Fennie, D. B. Powers, and D. A. Estell. 1984. Synergistic antiviral and antiproliferative activities of Escherichia coli-derived human alpha, beta, and gamma interferons. J. Virol. 49:490-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis, T. M. E. 2004. Mefloquine, p. 1091-1104. In V. L. Yu, G. Edwards, P. S. McKinnon, C. Peloquin, and G. D. Morse (ed.), Antimicrobial therapy and vaccines, vol. II. Antimicrobial agents. Apple Trees Productions, New York, N.Y. [Google Scholar]
- 8.Davis, T. M. E., T. Y. Hung, I. K. Sim, H. A. Karunajeewa, and K. F. Ilett. 2005. Piperaquine: a resurgent antimalarial drug. Drugs 65:75-87. [DOI] [PubMed] [Google Scholar]
- 9.Davis, T. M. E., H. A. Karunajeewa, and K. F. Ilett. 2005. Artemisinin-based combination therapies for uncomplicated malaria. Med. J. Aust. 182:181-185. [DOI] [PubMed] [Google Scholar]
- 10.Denis, M. B., T. M. E. Davis, S. Hewitt, S. Incardona, K. Nimol, T. Fandeur, Y. Poravuth, C. Lim, and D. Socheat. 2002. Efficacy and safety of dihydroartemisinin-piperaquine (Artekin) in Cambodian children and adults with uncomplicated falciparum malaria. Clin. Infect. Dis. 35:1469-1476. [DOI] [PubMed] [Google Scholar]
- 11.De Vries, P. J., and T. K. Dien. 1996. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs 52:818-836. [DOI] [PubMed] [Google Scholar]
- 12.Geary, T. G., A. D. Divo, J. B. Jensen, M. Zangwill, and H. Ginsburg. 1990. Kinetic modelling of the response of P. falciparum to chloroquine and its experimental testing in vitro. Implications for mechanism of action of and resistance to the drug. Biochem. Pharmacol. 40:685-691. [DOI] [PubMed] [Google Scholar]
- 13.Gupta, S., M. M. Thapar, S. T. Mariga, W. H. Wernsdorfer, and A. Bjorkman. 2002. Plasmodium falciparum: in vitro interactions of artemisinin with amodiaquine, pyronaridine, and chloroquine. Exp. Parasitol. 100:28-35. [DOI] [PubMed] [Google Scholar]
- 14.Hamzah, J., T. Skinner-Adams, and T. M. E. Davis. 2003. In vitro antimalarial activity of retinoids and the influence of selective retinoic acid receptor antagonists. Acta Trop. 87:345-353. [DOI] [PubMed] [Google Scholar]
- 15.Huber, W., and J. C. Koella. 1993. A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop. 55:257-261. [DOI] [PubMed] [Google Scholar]
- 16.Jambou, R., E. Legrand, M. Niang, N. Khim, P. Lim, B. Volney, M. T. Ekala, C. Bouchier, P. Esterre, T. Fandeur, and O. Mercereau-Puijalon. 2005. Resistance of Plasmodium falciparum field isolates to in-vitro artemether and point mutations of the SERCA-type PfATPase6. Lancet 366:1960-1963. [DOI] [PubMed] [Google Scholar]
- 17.Odds, F. C. 2003. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. [DOI] [PubMed] [Google Scholar]
- 18.Rahman, N. N. 1997. Evaluation of the sensitivity in vitro of Plasmodium falciparum and in vivo of Plasmodium chabaudi malaria to various drugs and their combinations. Med. J. Malaysia 52:390-398. [PubMed] [Google Scholar]
- 19.Rosenblum, M. G. 1991. Biochemical effects of human alpha interferon in combination with alpha-difluromethylornithine on human lymphoblastoid (DAUDI) cells in culture. Lymphokine Cytokine Res. 10:83-87. [PubMed] [Google Scholar]
- 20.Skinner-Adams, T., and T. M. E. Davis. 1999. Synergistic in vitro antimalarial activity of omeprazole and quinine. Antimicrob. Agents Chemother. 43:1304-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stahel, E., P. Druilhe, and M. Gentilini. 1988. Antagonism of chloroquine with other antimalarials. Trans. R. Soc. Trop. Med. Hyg. 82:221. [DOI] [PubMed] [Google Scholar]
- 22.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 23.Tran, T. H., C. Dolecek, P. M. Pham, T. D. Nguyen, T. T. Nguyen, H. T. Le, T. H. Dong, T. T. Tran, K. Stepniewska, N. J. White, and J. Farrar. 2004. Dihydroartemisinin-piperaquine against multidrug-resistant Plasmodium falciparum malaria in Vietnam: randomised clinical trial. Lancet 363:18-22. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. 2001. Antimalarial drug combination therapy. Report of a WHO technical consultation. WHO/CDS/RBM/2001.35. WHO, Geneva, Switzerland.