Abstract
Filamentous actinomycetes are commercially widely used as producers of natural products (in particular antibiotics) and of industrial enzymes. However, the mycelial lifestyle of actinomycetes, resulting in highly viscous broths and unfavorable pellet formation, has been a major bottleneck in their commercialization. Here we describe the successful morphological engineering of industrially important streptomycetes through controlled expression of the morphogene ssgA. This led to improved growth of many industrial and reference streptomycetes, with fragmentation of the mycelial clumps resulting in significantly enhanced growth rates in batch fermentations of Streptomyces coelicolor and Streptomyces lividans. Product formation was also stimulated, with a twofold increase in yield of enzyme production by S. lividans. We anticipate that the use of the presented methodology will make actinomycetes significantly more attractive as industrial and sustainable production hosts.
The exponential increase in the global demand for natural products and bulk chemicals effects an increasing burden on the environment, necessitating more sustainable fermentation technologies (6). Filamentous microorganisms are widely used as industrial producers of products such as antibiotics, anticancer agents, antifungicides, and enzymes (2, 8, 12). These organisms include the eukaryotic filamentous fungi (ascomycetes) and the prokaryotic actinomycetes (e.g., Amycolatopsis, Nocardia, and Streptomyces). The market capitalizations for antibiotics and enzymes total around 28 and 2 billion dollars per year, respectively. The study of genome sequences of actinomycetes has unveiled a surprisingly large number of cryptic antibiotic biosynthesis clusters, thus offering new challenges for directed drug discovery (11). A novel screening technique established that selective growth conditions can induce the normally dormant biosynthetic clusters for enediyne-type antitumor antibiotics (25). In contrast to unicellular microorganisms such as Escherichia coli, Bacillus spp., and Saccharomyces cerevisiae, filamentous microbes are highly heteromorphous and are therefore less favorable production organisms. The mycelial lifestyle of actinomycetes results in production processes that are typically characterized by a complex rheology (1, 7, 10). This results in mass-related mechanical stress, in heat transfer problems, and in oxidative stress (16, 17). The consequential problems related to filamentous growth include (i) slow growth rates and highly viscous cultures, (ii) large mycelial clumps that are mainly physiologically active around the edge of the clump (pellet), with significant oxygen and nutrient transfer problems towards the center, (iii) mixing requirements that necessitate high stirrer speeds, resulting in uncontrolled fragmentation and lysis of the mycelium, and (iv) complex and therefore expensive downstream processing. While enzymes from actinomycetes are often produced in an industrially preferred host (plug-bug approach), other enzymes and (almost) all antibiotics can only be synthesized by the natural producer. For antibiotics this is primarily due to the complexity of the biosynthesis clusters (12), and for proteins it is due to the actinomycete-specific sorting sequences that are not recognized by other bacterial secretion systems (9). Better understanding of factors that control growth and morphology is therefore a prerequisite for the optimization of production processes. In-depth studies by Bushell and colleagues on erythromycin production by Saccharopolyspora erythraea showed a striking correlation between the diameter of the mycelium fragments and productivity (7). Interestingly, selection of variants with reduced branching rates and enhanced strength of the cell wall resulted in a significantly enhanced production (23). It is unclear how these observations translate to other production processes (e.g., enzymes), as antibiotic production is often strongly growth phase dependent (4). The molecular mechanisms underlying morphology of actinomycetes in liquid-grown cultures are only poorly understood. However, at least one important factor is SsgA, a member of a novel family of actinomycete-specific proteins with six or seven members in streptomycetes that relate functionally to cell division and morphogenesis (18). The SsgA-like proteins appear to be primarily involved in the control of peptidoglycan maintenance. It was shown that the growth behavior of streptomycetes in submerged cultures depends in part on the expression level of ssgA, whereby enhanced SsgA protein levels result in mycelial fragmentation (14, 21, 22).
In this paper we show that the fragmentation effected by the enhanced expression of ssgA results in faster growth in batch fermentations of several streptomycetes, resulting in shorter fermentation times and improved productivity.
MATERIALS AND METHODS
Bacterial strains, fermentations, and culturing conditions.
The Streptomyces species described in this paper were S. lividans 1326 and S. coelicolor A3(2) M145, which were obtained from the John Innes Centre strain collection, and S. limosus ISP5131 (ATCC 19778), S. rimosus NRRL2234 (ATCC 10970), S. roseosporus NRRL11379 (ATCC 31568), and S. venezuelae ATCC 15439, obtained from the American Type Culture Collection (ATCC). Modified strains harboring plasmid pGWS4-SD, which integrates at the ϕC31 attachment site and gives overproduction of the SsgA protein (21), were designated S. lividans GSAL1 and S. coelicolor GSA2. Control strains harbored pSET152 (5) without insert.
Larger-scale fermentations were performed in a 42-liter Applikon fermentor. The defined (minimal) medium, as well as preculturing and inoculation conditions, was described earlier (19), except that glycerol was replaced with an equimolar amount of glucose. The fermentations were performed at 30°C, and the pH was controlled at 7.00 by addition of 2 M H2SO4 or 2 M NaOH. Glutamate utilization was inferred from the acid addition required to neutralize the released ammonia. To validate our data, the exact glutamate concentration was determined enzymatically at several time points by use of a glutamate dehydrogenase-diaphorase-coupled assay (19). The stirrer speed was maintained at 500 rpm, and the dissolved oxygen tension was kept above 50% by increasing the overpressure and blending in oxygen in the gas inlet. The inlet and outlet gas compositions were measured using a VG Gas Prima 600 mass spectrometer.
Small-scale fermentations for the analysis of productivity of modified streptomycetes were performed in a BioFlo 3000 5-liter bench-top fermentor (New Brunswick Biosciences). Fermentations were performed at 30°C, and the pH was controlled at 6.7 by the addition of 2 N phosphoric acid or 2 N NaOH. Dissolved oxygen tension was set at 80% and maintained by changing the stirrer speed. S. coelicolor M145 and GSA2 were grown in 4 liters of YEME medium (15) without sucrose, and S. lividans 1326 and GSAL1 harboring pIJ703 (13) were grown in 4 liters of tryptone soy broth with 10% sucrose (TSBS) containing 2.5 μg/ml thiostrepton (to maintain the plasmid) and 25 μM CuCl2. All fermentations were inoculated with 100 ml from a preculture grown in the same medium as the final broth for 30 h at 30°C in a spring-coiled flask. All fermentations were carried out three times to ensure reproducibility.
Tyrosinase activity assays.
The specific enzymatic activity of tyrosinase secreted by transformants of S. lividans 1326 harboring pIJ703 (13) was determined as described previously, by following the conversion of l-3,4-dihydroxyphenylalanine spectrophotometrically (15a) and by determining the biomass content (dry weight) of the samples.
Antibiotic activity assays.
Actinorhodin production by S. coelicolor was determined as follows. Culture supernatant (4 ml) was treated with 50 μl 5 M HCl to pH 2 to 3, extracted with a 0.5 volume of methanol-chloroform (1:1), and centrifuged at 5,000 rpm for 10 min. The concentration was calculated from the A542 (ɛ542, 18,600). For measurement of undecylpriodigiosin, mycelia were extracted with methanol and acidified by addition of HCl to a 0.5 M final concentration, and the concentration was calculated from the A530 (ɛ530, 100,500).
DNA techniques.
Streptomyces transformations were performed according to methods described in reference 15, and selected transformants were checked for correctness by PCR with Pfu polymerase (Stratagene, La Jolla, CA), according to methods described in reference 20.
Microscopy.
Phase-contrast micrographs were taken with a Zeiss Standard 25 microscope and a high-resolution charge-coupled device camera. Photographs were processed with Adobe Photoshop CS software.
RESULTS
Effect of SsgA on industrial streptomycetes.
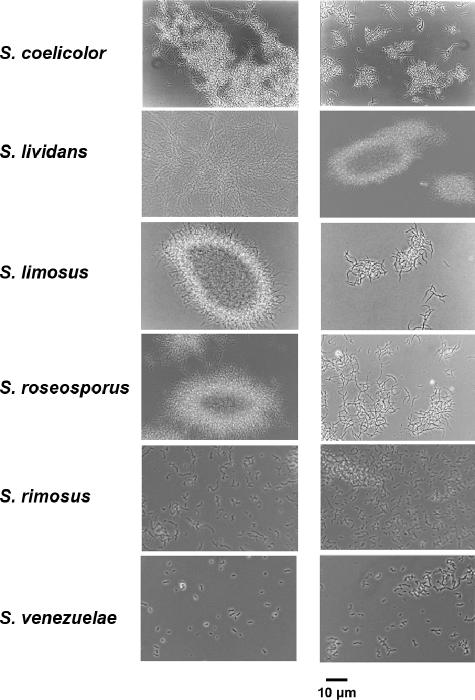
Previous work on the morphological effects of enhanced expression of ssgA showed that fragmented growth of mycelia was stimulated, suggesting possible advantages for industrial fermentations (14, 21). Closer inspection of S. coelicolor GSA2 (which overexpresses the SsgA protein) by transmission electron microscopy revealed a strong increase in septum formation (21). To assess the potential for growth improvement for industrial fermentations, the effects of the enhanced expression of ssgA on the morphology, growth, and productivity of streptomycetes were analyzed. A construct overproducing SsgA was introduced into the model system S. coelicolor A3(2) and the industrial Streptomyces species S. lividans (the preferred host for industrial enzyme production), S. limosus (a producer of amylases), S. rimosus (a producer of oxytetracycline), S. roseosporus (a producer of daptomycin), and S. venezuelae (a producer of chloramphenicol) (Fig. 1). The reproducible tendency was that enhanced expression of ssgA resulted in fragmented growth of strains that normally grow as pellets (S. coelicolor, S. lividans, and S. roseosporus). Repeated growth measurements of S. coelicolor and S. lividans (detailed below) and of S. roseosporus (see Fig. S1 in the supplemental material) revealed that the specific growth rates of the SsgA-modified strains had increased significantly in comparison to those of the parental strains. In the case of S. limosus, the parental strain produced large mycelial mat structures, while the enhanced expression of ssgA resulted in pellet formation. The effects of the morphological changes on growth and product formation were not analyzed. For S. rimosus and S. venezuelae, the parental strain already grew in a highly fragmented manner (S. rimosus) or even sporulated during fermentation (S. venezuelae), and for both strains we hardly observed mycelia larger than a few micrometers. Expectedly, SsgA had no noticeable effect on the morphology of S. rimosus; however, its enhanced expression prevented the sporulation of S. venezuelae, which is an obvious advantage under production conditions.
FIG. 1.
Effect of SsgA on morphology: representative phase-contrast micrographs of mycelia from various streptomycetes with control plasmid pSET152 (left) or with pGWS4-SD overproducing SsgA (right). For ATCC strain designations see Materials and Methods. Notice the strongly reduced pellet formation in S. coelicolor, S.lividans, and S. roseosporus due to the overexpression of ssgA. Bar, 10 μm.
Batch fermentation of S. coelicolor overproducing SsgA.
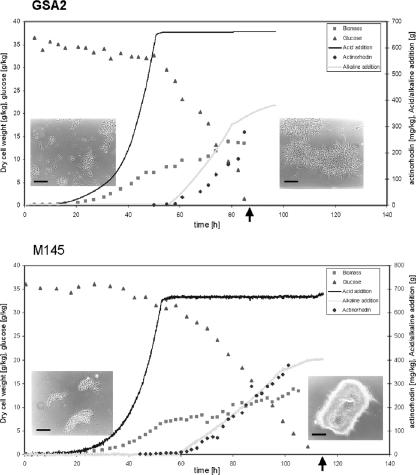
How do the morphological changes translate to changes in growth and product formation? To answer this we analyzed growth rates and productivity of S. coelicolor and S. lividans. We performed well-controlled batch fermentations on S. coelicolor M145, genetically the best-characterized actinomycete and with a known genome sequence (3). The growth-related problems are a major reason why S. coelicolor has never been used in large-scale industrial fermentations. We compared S. coelicolor GSA2 (overproducing SsgA) and its parent M145 harboring control plasmid pSET152. Both strains were first monitored in well-controlled 25-liter fermentations in defined minimal medium. While the parental strain produced the typical large pellets, especially towards the end of the fermentation, the enhanced expression of ssgA resulted in much smaller mycelial structures with many protruding hyphae (Fig. 2). During the first 50 h, glutamate was utilized for growth, while glucose consumption was minimal (Fig. 2). At this stage, the growth rates of GSA2 and M145/pSET152 were comparable. The transition from glutamate to glucose is marked by cessation of the requirement for acid addition (initially required to neutralize the secreted ammonium produced from glutamate). After glutamate depletion, growth continued on glucose and ammonium and production of the pigmented antibiotic actinorhodin was induced. The production profile of actinorhodin was similar in S. coelicolor M145 and GSA2 and, expectedly, ceased once the glucose was fully consumed. Interestingly, the growth-optimized strain GSA2 utilized glucose much more efficiently than M145 (depleting it within 35 h rather than 70 h), resulting in the end of fermentation after 85 h for GSA2 and after 115 h for its parent, M145. We measured and calculated the specific growth rate (μ), which was reproducible in several independent fermentations and averaged 0.14 h−1 for S. coelicolor M145 harboring control plasmid pSET152 and 0.20 h−1 for the ssgA-modified strain GSA2 in the defined (minimal) medium, or a 43% increase for μGSA2 versus μM145. Hence, the SsgA-induced morphological changes translated to a significant enhancement of the growth rate.
FIG. 2.
Effect of enhanced SsgA production on growth of S. coelicolor in a pilot-scale fermentor. Results shown are from batch fermentation of SsgA-overexpressing S. coelicolor GSA2 (top) and its parent M145, harboring control plasmid pSET152 (bottom), performed in a 42-liter fermentor. Glutamate was consumed prior to glucose, and glucose consumption started after approximately 50 h. Glutamate concentrations were calculated from the amount of added acid required to neutralize the ammonia produced from the utilization of glutamate. Growth ceased when glucose was fully consumed; this was after 85 h for GSA2 and after 115 h for M145 (indicated by arrows). Morphology of the mycelia after preculturing (pre) and after fermentation (end) is shown in the inset photographs. Clearly visible is the reduced pellet size in the GSA2 transformant. Bar, 20 μm.
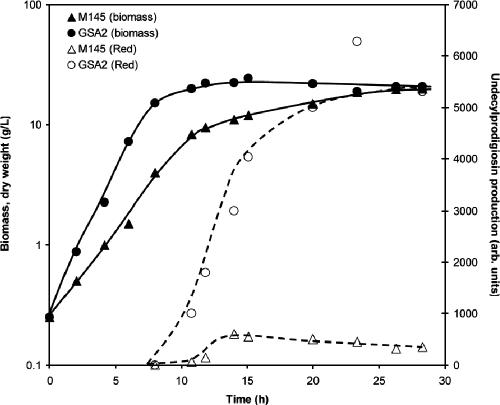
Subsequently, S. coelicolor GSA2 and M145 were grown in 4-liter batch fermentations in TSBS medium. Cultures were inoculated from precultures grown for 24 h to a starting biomass of 0.3 g/liter. On average, GSA2 reached a biomass concentration of 2.5 g/liter in 3.5 h, compared to 7 h for the same biomass for the parental strain, M145 (Fig. 3). The specific growth rate increased by 67%, from 0.33 h−1 (M145) to 0.55 h−1 (GSA2). While eventually the cultures reached approximately the same final biomass concentration (25 g [dry weight]/liter), GSA2 reached the stationary phase after only 7 h, while M145 control cultures entered the stationary growth phase after 12 h. In repeated fermentations under the same conditions, the specific growth rate of GSA2 was between 61 and 76% higher than that of the parental M145 strain. Production of the second pigmented antibiotic undecylprodigiosin reached a maximum of approximately 5,300 arbitrary units in GSA2 and only around 500 in M145, a difference of an order of magnitude (Fig. 3).
FIG. 3.
Effect of enhanced expression of ssgA on growth and antibiotic production of S. coelicolor. Batch fermentation of SsgA-overexpressing S. coelicolor GSA2 and its parent M145 (with control plasmid pSET152) was performed in TSBS medium, with control of pH (maintained at 6.7) and of dissolved oxygen concentration (maintained at 80%). Biomass accumulation of S. coelicolor M145 (▴) and GSA2 (•) was calculated from dry weight measurements, while undecylprodigiosin (Red) production (open symbols) was measured spectrophotometrically.
SsgA enhances growth rate and tyrosinase production by S. lividans.
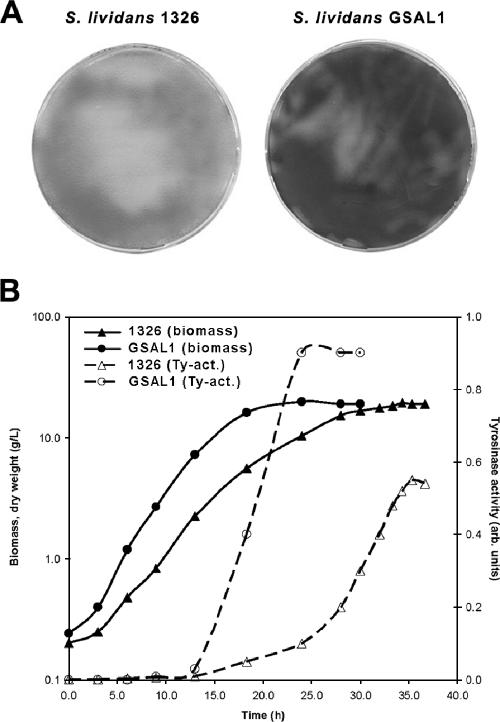
The morphological engineering technology was then applied to Streptomyces lividans, the preferred Streptomyces host for industrial enzyme production. We tested the SsgA-induced effects of the morphological changes on the yield of secreted enzymes in 4-liter fermentations in TSBS medium, using production of the phenoloxidase tyrosinase as the reference system. Therefore, pIJ703, a plasmid expressing the tyrosinase gene melC2 (13), was introduced into S. lividans 1326 and the SsgA-overproducing GSAL1. As expected, enhanced expression of ssgA altered the morphology of S. lividans, resulting in smaller clumps (Fig. 1). Overproduction of SsgA in S. lividans had a strongly positive effect on both growth rate and enzyme production (Fig. 4A and B). This was already apparent from the initial transformation plates, where colonies of S. lividans GSAL1 produced much more of the black-pigmented melanin (the product of the conversion of tyrosine by tyrosinase) than the parental 1326 (Fig. 4A). The observed maximum specific growth rate of S. lividans 1326 increased by 45% from 0.20 h−1 (strain 1326) to 0.29 h−1 (GSAL1) (Fig. 4B). Enzyme production was strongly enhanced, with a maximum tyrosinase level of 0.94 (relative units) around 20 h after the start of the fermentation for S. lividans GSAL1, compared to only 0.55 (relative units) after almost 35 h for the control strain. This pilot system showed that tyrosinase productivity (enzyme units/fermentation time) had increased by a factor of 2.5, which points to a strong improvement of productivity.
FIG. 4.
Effects of enhanced expression of ssgA on growth and tyrosinase production of S. lividans. (A) Tyrosinase production by S. lividans. S. lividans 1326 containing pIJ703 was transformed with pGWS4-SD (ssgA overproduction; strain GSAL1) or with pSET152 (control plasmid; strain 1326), and transformants were selected on R2YE agar plates. Representative plates with around 500 colonies are shown. Note the strongly enhanced tyrosinase production by the SsgA-overproducing transformant. As follows from the data in panel B, the SsgA-overproducing strain produced two to three times more tyrosinase than the control strain. Hence, enhanced expression of ssgA resulted in an important improvement of the tyrosinase yield. (B) Batch fermentation of SsgA-overproducing S. lividans GSAL1/pIJ703 and its parent, 1326/pIJ703 (harboring control plasmid pSET152), was performed in 4 liters of TSBS in a 5-liter fermentor. The pH was maintained at 6.7, and the dissolved oxygen concentration was maintained at 80%. Biomass accumulation of S. lividans 1326 (▴) and GSAL1 (•) was calculated from dry weight measurements. Tyrosinase production (for description of the assay, see Materials and Methods) is represented by the open symbols.
DISCUSSION
In this paper we established that enhanced fragmentation, such as that induced by increased expression of ssgA, has a major impact on growth and product formation by streptomycetes and is therefore expected to have an impact on biotechnological applications requiring Streptomyces as the production host. The morphology of liquid-grown mycelia is dictated by both external factors (medium and fermentation conditions) and genetic factors (e.g., the ssgA expression level). For example, we have established in many actinomycetes that the degree of fragmentation (the major determinant of mycelial clump size) is directly proportional to the frequency of septation (not shown). This explains why enhanced levels of SsgA, which stimulates primarily the formation of septa, induce fragmentation. It is likely that controlled fragmentation occurs at the septa, a process that is stimulated by weakening of the lateral walls of the vegetative hyphae, e.g., due to an acidic environment (not shown). Such controlled breakage at septa is advantageous, as here the hypha is divided into two compartments, each surrounded by a stable cell wall, and hence breakage at this position results in two viable daughters. In the case of uncontrolled fragmentation at a different (nonseptal) position, e.g., due to stirrer-induced mechanical shear, the compartment will break open, resulting in cell death.
Ideally, precultures should contain fragmented mycelia, so that the preculture contains a maximal number of growth nuclei. This allows low viscosity and optimal transfer of nutrients and oxygen due to the smaller mycelium size. Studies on erythromycin production by Saccharopolyspora erythraea showed a striking correlation between mycelium fragment diameter and productivity for this organism, with a critical fragment size (close to 100 μm) below which productivity was strongly reduced (7). Interestingly, selection of variants with reduced branching rates and enhanced strength of the cell wall resulted in significantly enhanced production (23). It is unclear how these observations translate to other production processes (e.g., enzymes), as antibiotic production is often strongly growth phase dependent (4).
An obvious and important question that we addressed in this work is, how does fragmentation translate to the production process, i.e., growth rate and yield of product formation? Interestingly, we observed that enhanced expression of ssgA in S. coelicolor, S. lividans, and S. roseosporus resulted in improved growth rates, with a reduced lag phase and a smaller average mycelium size. In 25-liter batch fermentations with a defined minimal medium, the specific growth rate increased from 0.14 h−1 to 0.20 h−1, a 43% increase. This positive effect on growth rate was confirmed by the 4-liter batch fermentations, where enhancement of the specific growth rate by the overexpression of ssgA was 67% (0.33 h−1 to 0.55 h−1) for S. coelicolor M145 and 45% (0.20 h−1 to 0.29 h−1) for S. lividans 1326. The differences in growth rates between 25-liter and 4-liter fermentations are explained by the media used (minimal versus rich). Eventually, cultures reached the same total biomass, but the obvious advantage was that for ssgA-modified strains the fermentation time was at least halved with respect to that required for the control strain. S. lividans is an important commercial strain, as it is used as the preferred Streptomyces host for enzyme production. Our pilot system showed that tyrosinase productivity (enzyme units/fermentation time) had increased by a factor of 2.5, which promises strong improvement of productivity. In a recent publication it was shown that also multiple copies of the AraC-type transcriptional regulator AdpA effect improved tyrosinase production (26). Most likely this also relates to the activity of SsgA, since AdpA is the transcriptional activator of ssgA in S. griseus (24). Besides the effect on enzyme production/secretion by S. lividans, in cultures of S. coelicolor GSA2 we observed a strong increase in undecylprodigiosin production (approximately 10-fold). However, actinorhodin production was somewhat reduced in the modified strain. The latter is explained by the rapid consumption of glucose by GSA2 in the final growth phase in comparison to the slower-growing parental strain.
In conclusion, the enhanced expression of ssgA may prove an efficient means for the directed strain improvement of actinomycetes that are difficult to cultivate, while the more fragmented growth also makes growth in microtiter plates much more feasible and an important issue for high-throughput screening. Streptomycetes produce a wealth of natural products, both secondary metabolites and enzymes, and the application of SsgA as a means to improve their growth and productivity will possibly make them a more attractive alternative to currently used commercial production hosts. Besides these attractive cost-related aspects, the reduced energy requirement offers new means in the battle for more sustainable production solutions.
Supplementary Material
Acknowledgments
We are grateful to F. Goedegebuur, J. van der Laan, R. Luiten, H. de Nobel, and J. Texeira de Mattos for stimulating discussions.
This work was supported by grants from the Research Council for Chemical Sciences (C.W.) with financial aid from The Netherlands Technology Foundation to B.K. and J.J.H., from BioPartner to G.P.V.W. and E.V., and from the Royal Netherlands Academy of Arts and Sciences to G.P.V.W.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Bai, Z., L. M. Harvey, and B. McNeil. 2003. Oxidative stress in submerged cultures of fungi. Crit. Rev. Biotechnol. 23:267-302. [DOI] [PubMed] [Google Scholar]
- 2.Bennett, J. W. 1998. Mycotechnology: the role of fungi in biotechnology. J. Biotechnol. 66:101-107. [DOI] [PubMed] [Google Scholar]
- 3.Bentley, S. D., K. F. Chater, A. M. Cerdeno-Tarraga, G. L. Challis, N. R. Thomson, K. D. James, D. E. Harris, M. A. Quail, H. Kieser, D. Harper, A. Bateman, S. Brown, G. Chandra, C. W. Chen, M. Collins, A. Cronin, A. Fraser, A. Goble, J. Hidalgo, T. Hornsby, S. Howarth, C. H. Huang, T. Kieser, L. Larke, L. Murphy, K. Oliver, S. O'Neil, E. Rabbinowitsch, M. A. Rajandream, K. Rutherford, S. Rutter, K. Seeger, D. Saunders, S. Sharp, R. Squares, S. Squares, K. Taylor, T. Warren, A. Wietzorrek, J. Woodward, B. G. Barrell, J. Parkhill, and D. A. Hopwood. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141-147. [DOI] [PubMed] [Google Scholar]
- 4.Bibb, M. 1996. 1995 Colworth Prize lecture. The regulation of antibiotic production in Streptomyces coelicolor A3(2). Microbiology 142:1335-1344. [DOI] [PubMed] [Google Scholar]
- 5.Bierman, M., R. Logan, K. O'Brien, E. T. Seno, R. N. Rao, and B. E. Schoner. 1992. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43-49. [DOI] [PubMed] [Google Scholar]
- 6.Bruggink, A., A. J. Straathof, and L. A. van der Wielen. 2003. A ‘fine’ chemical industry for life science products: green solutions to chemical challenges. Adv. Biochem. Eng. Biotechnol. 80:69-113. [DOI] [PubMed] [Google Scholar]
- 7.Bushell, M. E. 1988. Growth, product formation and fermentation technology, p. 185-217. In M. Goodfellow, S. T. Williams, and M. Modarski (ed.), Actinomycetes in biotechnology. Academic Press, London, United Kingdom.
- 8.Demain, A. L. 1991. Production of beta-lactam antibiotics and its regulation. Proc. Natl. Sci. Council Repub. China B 15:251-265. [PubMed] [Google Scholar]
- 9.Gabor, E. M., W. B. Alkema, and D. B. Janssen. 2004. Quantifying the accessibility of the metagenome by random expression cloning techniques. Environ. Microbiol. 6:879-886. [DOI] [PubMed] [Google Scholar]
- 10.Gomes, J., and A. S. Menawat. 1998. Fed-batch bioproduction of spectinomycin. Adv. Biochem. Eng. Biotechnol. 59:1-46. [DOI] [PubMed] [Google Scholar]
- 11.Hopwood, D. A. 2003. The Streptomyces genome—be prepared! Nat. Biotechnol. 21:505-506. [DOI] [PubMed] [Google Scholar]
- 12.Hopwood, D. A., K. F. Chater, and M. J. Bibb. 1995. Genetics of antibiotic production in Streptomyces coelicolor A3(2), a model streptomycete. Biotechnology 28:65-102. [DOI] [PubMed] [Google Scholar]
- 13.Katz, E., C. J. Thompson, and D. A. Hopwood. 1983. Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J. Gen. Microbiol. 129:2703-2714. [DOI] [PubMed] [Google Scholar]
- 14.Kawamoto, S., H. Watanabe, A. Hesketh, J. C. Ensign, and K. Ochi. 1997. Expression analysis of the ssgA gene product, associated with sporulation and cell division in Streptomyces griseus. Microbiology 143:1077-1086. [DOI] [PubMed] [Google Scholar]
- 15.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. John Innes Foundation, Norwich, United Kingdom.
- 15a.Lerch, K., and L. Ettinger. 1972. Purification and characterization of a tyrosinase from Streptomyces glaucescens. Eur. J. Biochem. 31:427-437. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen, J. 1996. Modelling the morphology of filamentous microorganisms. Trends Biotechnol. 14:438-443. [Google Scholar]
- 17.Nielsen, J., C. L. Johansen, M. Jacobsen, P. Krabben, and J. Villadsen. 1995. Pellet formation and fragmentation in submerged cultures of Penicillium chrysogenum and its relation to penicillin production. Biotechnol. Prog. 11:93-98. [DOI] [PubMed] [Google Scholar]
- 18.Noens, E. E., V. Mersinias, B. A. Traag, C. P. Smith, H. K. Koerten, and G. P. van Wezel. 2005. SsgA-like proteins determine the fate of peptidoglycan during sporulation of Streptomyces coelicolor. Mol. Microbiol. 58:929-944. [DOI] [PubMed] [Google Scholar]
- 19.Roubos, J. A., P. Krabben, R. G. Luiten, H. B. Verbruggen, and J. J. Heijnen. 2001. A quantitative approach to characterizing cell lysis caused by mechanical agitation of Streptomyces clavuligerus. Biotechnol. Prog. 17:336-347. [DOI] [PubMed] [Google Scholar]
- 20.Traag, B. A., G. H. Kelemen, and G. P. Van Wezel. 2004. Transcription of the sporulation gene ssgA is activated by the IclR-type regulator SsgR in a whi-independent manner in Streptomyces coelicolor A3(2). Mol. Microbiol. 53:985-1000. [DOI] [PubMed] [Google Scholar]
- 21.van Wezel, G. P., J. van der Meulen, S. Kawamoto, R. G. M. Luiten, H. K. Koerten, and B. Kraal. 2000. ssgA is essential for sporulation of Streptomyces coelicolor A3(2) and affects hyphal development by stimulating septum formation. J. Bacteriol. 182:5653-5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Wezel, G. P., J. van der Meulen, E. Taal, H. Koerten, and B. Kraal. 2000. Effects of increased and deregulated expression of cell division genes on the morphology and on antibiotic production of streptomycetes. Antonie Leeuwenhoek 78:269-276. [DOI] [PubMed] [Google Scholar]
- 23.Wardell, J. N., S. M. Stocks, C. R. Thomas, and M. E. Bushell. 2002. Decreasing the hyphal branching rate of Saccharopolyspora erythraea NRRL 2338 leads to increased resistance to breakage and increased antibiotic production. Biotechnol. Bioeng. 78:141-146. [DOI] [PubMed] [Google Scholar]
- 24.Yamazaki, H., Y. Ohnishi, and S. Horinouchi. 2003. Transcriptional switch on of ssgA by A-factor, which is essential for spore septum formation in Streptomyces griseus. J. Bacteriol. 185:1273-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zazopoulos, E., K. Huang, A. Staffa, W. Liu, B. O. Bachmann, K. Nonaka, J. Ahlert, J. S. Thorson, B. Shen, and C. M. Farnet. 2003. A genomics-guided approach for discovering and expressing cryptic metabolic pathways. Nat. Biotechnol. 21:187-190. [DOI] [PubMed] [Google Scholar]
- 26.Zhu, D., X. He, X. Zhou, and Z. Deng. 2005. Expression of the melC operon in several Streptomyces strains is positively regulated by AdpA, an AraC family transcriptional regulator involved in morphological development in Streptomyces coelicolor. J. Bacteriol. 187:3180-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.