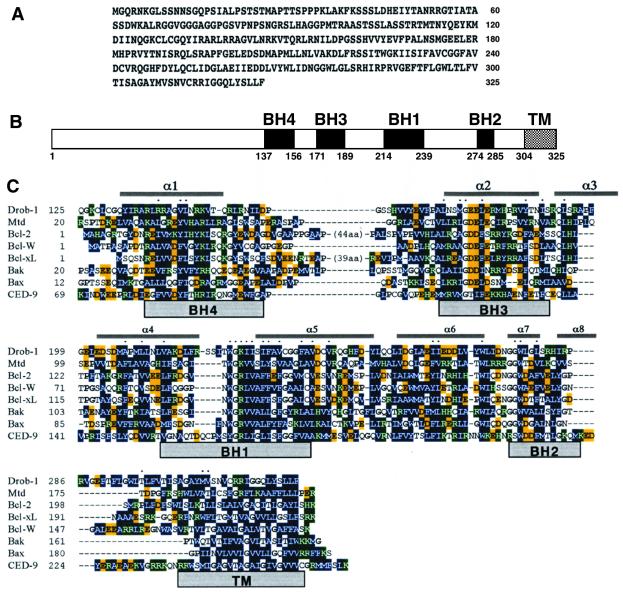
Figure 1.
Sequence and structure of Drob-1. (A) Deduced amino acid sequence of Drob-1. (B) Schematic structure of Drob-1. The conserved BH1, BH2, BH3, and BH4 regions and the putative transmembrane (TM) region are shown by closed boxes. (C) The amino acid sequence of Drob-1 is aligned with those of mouse Mtd, human Bcl-2, Bcl-xL, Bcl-w, Bak, Bax, and C. elegans CED-9. Hydrophobic residues are shown in dark blue. Negatively and positively charged residues are highlighted in yellow and green, respectively. Proline and glycine residues (α/β breakers) are in bold. Conserved residues are indicated by dots. Residues that form the flexible loop in Bcl-2 (44 aa) and Bcl-xL (39 aa) are not included for simplicity. A transmembrane domain predicted by the Kyte and Doolittle method is indicated as TM.