Figure 5.
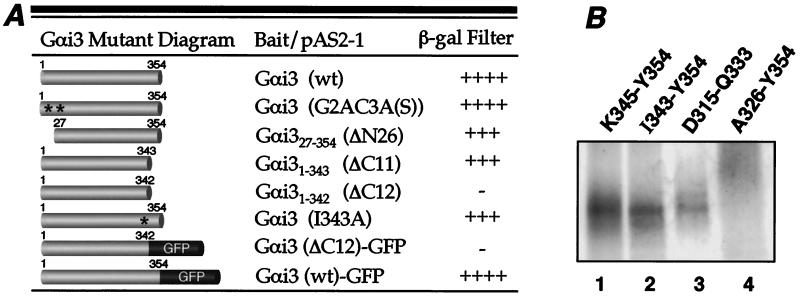
Calnuc interacts with the C-terminal α5-helix of Gαi3. (A) Two-hybrid assays. Gαi3 mutants were subcloned into the pAS2–1 bait vector, and calnuc was constructed in the pACT2 prey vector. The β-galactosidase filter assay was performed on (Leu−, Trp−) plates, and color was scored after 2 h. (−, negative; +, weak; ++, intermediate; +++, strong; ++++, very strong). Gαi3 mutants in which N-terminal sites for both myristoylation and palmitoylation were mutated [G2AC3A(S)] or the first 26 amino acids were truncated (ΔN26) bind to calnuc as strongly as wild-type (wt) Gαi3. Similarly, truncation of the last 11 C-terminal amino acids (Gαi3 ΔC11) does not reduce Gαi3 binding to calnuc whereas the interaction is completely abolished by deletion of the last 12 C-terminal amino acids (Gαi3 ΔC12). However, Gαi3 (I343A) still strongly binds to calnuc. Addition of GFP to the Gαi3 mutant with the C-terminal 12 amino acids deleted (Gαi3 ΔC12-GFP) does not restore binding to calnuc. Full length Gαi3 (wt)-GFP shows strong interaction with calnuc. Reproducible results were obtained in three separate experiments. (B) Peptide competition assays. 2 mM of each of four synthetic peptides and in vitro translated [35S]Gαi3 were incubated with calnuc-Sepharose 4B in the presence of 15 mM Mg2+/2 mM Ca2+ as described in Fig. 2A, followed by SDS/PAGE and autoradiographic analysis. The peptides K345-Y354 (lane 1), I343-Y354 (lane 2), and D315-Q333 (lane 3) are not able to compete [35S]Gαi3 binding to calnuc whereas peptide A326-Y354 (lane 4) completely inhibits calnuc-Gαi3 interaction.