Abstract
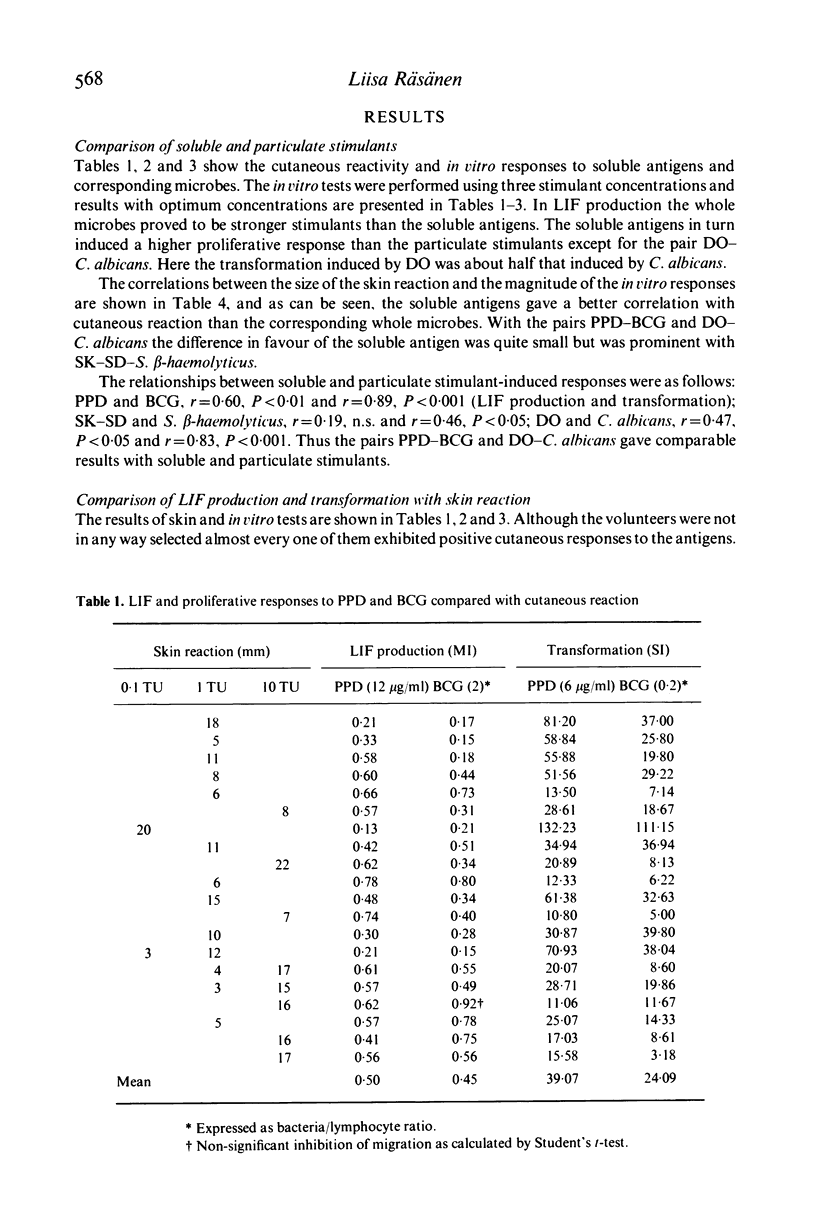
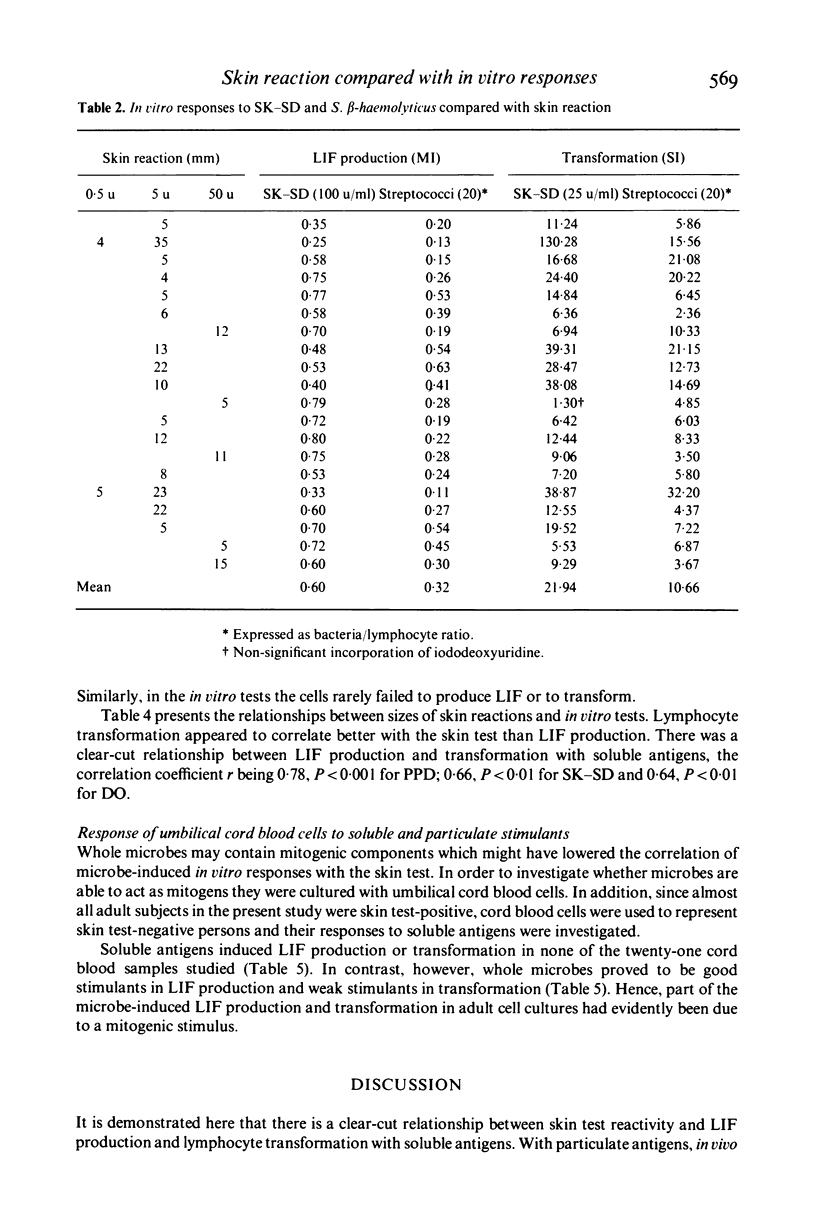
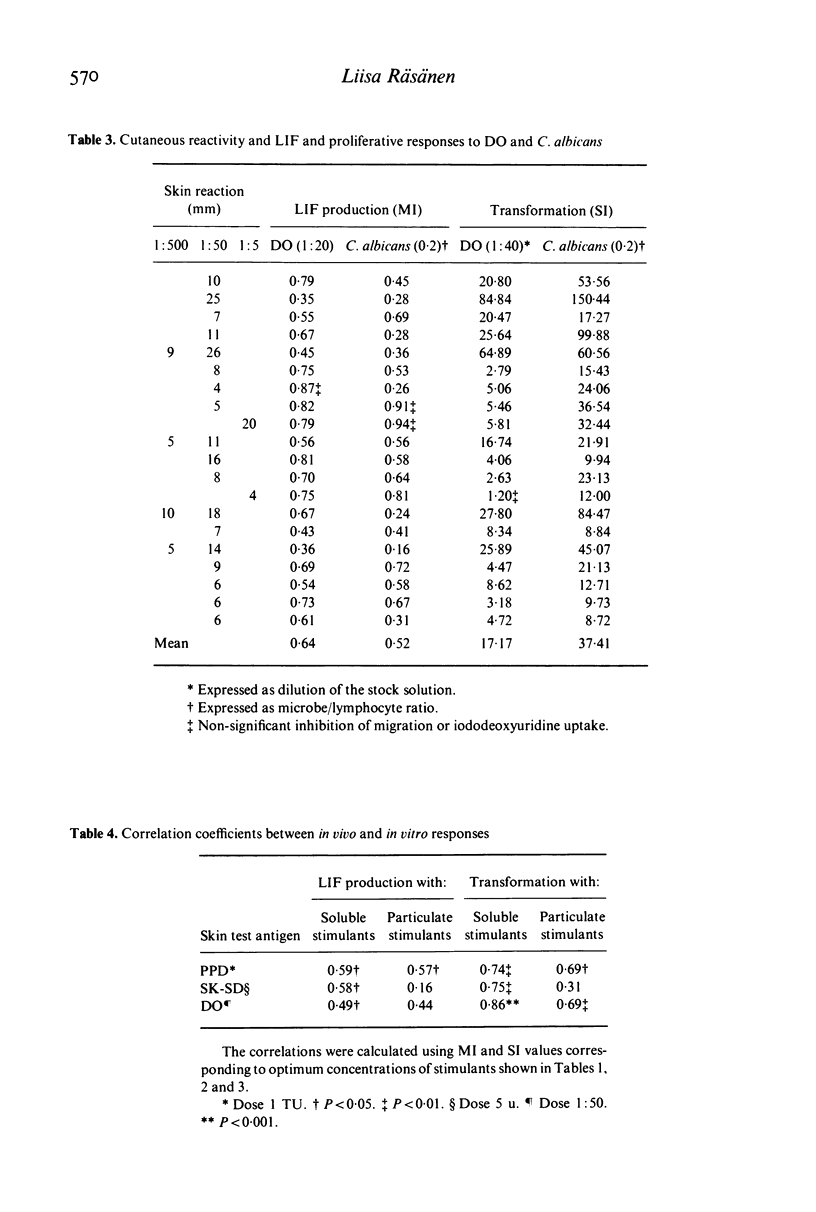
In the present study the relationships between skin reactivity, leucocyte migration inhibitory factor (LIF) production and lymphocyte transformation in healthy volunteers and newborns were investigated. LIF synthesis was assessed by the two-step agarose migration method and cellular proliferation by the incorporation of iododeoxyuridine. The following soluble antigens and whole microbes were used: purified protein derivative of tuberculin (PPD), bacille Calmette-Guérin (BCG), streptokinase-streptodornase (SK-SD), Streptococcus Beta-haemolyticus, group A, Dermatophytin O (DO) and Candida albicans. The results indicated a significant correlation between skin reactivity and in vitro tests with soluble antigens and that, of the in vitro tests used, lymphocyte transformation correlated better with skin test than LIF production. Furthermore, soluble antigens gave better correlations with skin tests than whole microbes. Experiments with cord blood cells demonstrated that they did not respond to antigens but were activated by whole microbes. Thus the whole microbes appear to contain mitogenic components.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders E. M., Natvig J. B. Cell-mediated immunity to viruses measured by the indirect agarose technique of leukocyte migration inhibition. Cell Immunol. 1976 Dec;27(2):214–229. doi: 10.1016/0008-8749(76)90230-6. [DOI] [PubMed] [Google Scholar]
- Asantila T., Toivanen P. Potentation by fluorodeoxyuridine of 125I-deoxyuridine uptake by human and chicken lymphocytes in the quantitation of mitogenic response. J Immunol Methods. 1974 Dec;6(1-2):73–82. doi: 10.1016/0022-1759(74)90091-x. [DOI] [PubMed] [Google Scholar]
- Bergstrand H., Källén B., Nilsson O. On the reactivity of human leukocytes to PPD in Clausen's agarose migration technique. Acta Allergol. 1974;29(2):117–135. doi: 10.1111/j.1398-9995.1974.tb01463.x. [DOI] [PubMed] [Google Scholar]
- Chapman S. W., Kirkpatrick C. H. The two-step leukocyte migration inhibition factor (LIF) assay. Its use in evaluation of cellular immune function in patients with immunodeficiency diseases. Cell Immunol. 1978 Apr;37(1):209–220. doi: 10.1016/0008-8749(78)90188-0. [DOI] [PubMed] [Google Scholar]
- Clausen J. E. Migration inhibitory effect of cell-free supernatants from tuberculin-stimulated cultures of human mononuclear leukocytes demonstrated by two-step MIF agarose assay. J Immunol. 1973 Feb;110(2):546–551. [PubMed] [Google Scholar]
- Clausen J. E. Tuberculin-induced migration inhibition of human peripheral leucocytes in agarose medium. Acta Allergol. 1971 Feb;26(1):56–80. doi: 10.1111/j.1398-9995.1971.tb01399.x. [DOI] [PubMed] [Google Scholar]
- Fleer A., van der Hart M., Blok-Schut B. J., Schellekens P. T. Correlation of PPD and BCG-induced leukocyte migration inhibition, delayed cutaneous hypersensitivity, lymphocyte transformation in vitro and humoral antibodies to PPD in man. Eur J Immunol. 1976 Mar;6(3):163–167. doi: 10.1002/eji.1830060305. [DOI] [PubMed] [Google Scholar]
- Górski A. J. Superiority of corpuscular BCG to soluble PPD antigen in the leucocyte migration assay. Clin Exp Immunol. 1974 Sep;18(1):149–153. [PMC free article] [PubMed] [Google Scholar]
- Hinz C. F., Jr, Daniel T. M., Baum G. L. Quantitative aspects of the stimulation of lymphocytes by tuberculin purified protein derivative. Int Arch Allergy Appl Immunol. 1970;38(2):119–129. doi: 10.1159/000230265. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Darai G., Hirt H. M., Keyssner K., Munk K. In vitro mitogenic stimulation of murine spleen cells by herpes simplex virus. J Immunol. 1978 Feb;120(2):641–645. [PubMed] [Google Scholar]
- Kirchner H., Tosato G., Blaese R. M., Broder S., Magrath I. T. Polyclonal immunoglobulin secretion by human B lymphocytes exposed to Epstein-Barr virus in vitro. J Immunol. 1979 Apr;122(4):1310–1313. [PubMed] [Google Scholar]
- Maini R. N., Roffe L. M., Magrath I. T., Dumonde D. C. Standardization of the leucocyte migration test. Int Arch Allergy Appl Immunol. 1973;45(1):308–321. doi: 10.1159/000231048. [DOI] [PubMed] [Google Scholar]
- McFarland W., Heilman D. H. Comparison of lymphocyte transformation and intradermal reactions to tuberculins. Am Rev Respir Dis. 1966 May;93(5):742–748. doi: 10.1164/arrd.1966.93.5.742. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Naot Y., Ginsburg H. Activation of B lymphocytes by mycoplasma mitogen(s). Immunology. 1978 Apr;34(4):715–720. [PMC free article] [PubMed] [Google Scholar]
- Nilsson B. S. The response of lymphocytes from tuberculin-positive or negative humans to various doses of PPD-tuberculin in vitro. Cell Immunol. 1972 Mar;3(3):493–500. doi: 10.1016/0008-8749(72)90254-7. [DOI] [PubMed] [Google Scholar]
- Räsänen L., Karhumäki E., Arvilommi H. Bacteria induce lymphokine synthesis polyclonally in human B lymphocytes. J Immunol. 1978 Aug;121(2):418–420. [PubMed] [Google Scholar]
- Senyk G., Hadley W. K. In vitro correlates of delayed hypersensitivity in man: ambiguity of polymorphonuclear neutrophils as indicator cells in leukocyte migration test. Infect Immun. 1973 Sep;8(3):370–380. doi: 10.1128/iai.8.3.370-380.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabriskie J. B., Falk R. E. In vitro reactivity of lymphocytes to particulate and soluble antigens. Nature. 1970 Jun 6;226(5249):943–945. doi: 10.1038/226943a0. [DOI] [PubMed] [Google Scholar]


