Abstract
An unusual effect of temperature on the ATPase activity of E. coli F1Fo ATP synthase has been investigated. The rate of ATP hydrolysis by the isolated enzyme, previously kept on ice, showed a lag phase when measured at 15° C, but not at 37° C. A pre-incubation of the enzyme at room temperature for 5 minutes completely eliminated the lag phase, and resulted in a higher steady-state rate. Similar results were obtained using the isolated enzyme after incorporation into liposomes. The initial rates of ATP-dependent proton translocation, as measured by 9-amino-6-chloro-2-methoxyacridine (ACMA) fluorescence quenching, at 15° C also varied according to the pre-incubation temperature. The relationship between this temperature-dependent pattern of enzyme activity, termed thermohysteresis, and pre-incubation with other agents was examined. Pre-incubation of membrane vesicles with azide and Mg2+, without exogenous ADP, resulted in almost complete inhibition of the initial rate of ATPase when assayed at 10° C, but had little effect at 37° C. Rates of ATP synthesis following this pre-incubation were not affected at any temperature. Azide inhibition of ATP hydrolysis by the isolated enzyme was reduced when an ATP-regenerating system was used. A gradual reactivation of azide-blocked enzyme was slowed down by the presence of phosphate in the reaction medium. The well-known Mg2+ inhibition of ATP hydrolysis was shown to be greatly enhanced at 15° C relative to at 37° C. The results suggest that thermohysteresis is a consequence of an inactive form of the enzyme that is stabilized by the binding of inhibitory Mg-ADP.
Keywords: F1Fo, ATP synthase, thermohysteresis, azide, Mg-ADP inhibition
1. Introduction
F1Fo-ATP synthases, present in mitochondria, chloroplasts and bacteria, catalyze ATP synthesis driven by proton or sodium translocation across the membrane. In some bacteria this enzyme can also function as an ion translocator driven by ATP hydrolysis. The enzyme consists of two distinct parts: F1, containing six nucleotide-binding sites and three catalytic centers, and membrane-embedded Fo responsible for proton translocation [1]. In the E. coli ATP synthase F1 is composed of five subunits in a stoichiometry of α3β3δε [2]. Fo consists of three subunits in a stoichiometry of ab2c10 [3, 4]. It is well established that the coupling between the ATP hydrolytic reaction and proton translocation is mediated by rotation of the central stalk εc10 (rotor) relative to the stator (α3β3ab2δ) [5-11]. F1 can be separated physically from Fo and it catalyses energy-uncoupled ATP hydrolysis at a high rate. The isolated Fo can act as a passive proton pore. Despite great success in studying this nano-motor, many mechanistic aspects of its function remain unknown. The E. coli ATP synthase is among the simplest of those that have been studied. Studies of the homologous enzymes from Bacillus PS3, P. modestum and I. tartaricus have also contributed to our understanding of ATP synthesis [9, 12, 13]. These enzymes share many, but certainly not all, features with the more complex enzymes found in mitochondria.
A common feature of ATP synthases is that the soluble F1-ATPases, but not the whole complex F1Fo, undergoes cold inactivation accompanied by dissociation of the enzyme into sub-complexes [14-16]. The intact F1Fo complex is less sensitive to incubation at low temperature, and its sensitivity to the cold has been interpreted as mediated by dissociation of F1 from the membrane and subsequent cold inactivation of the soluble F1 [14]. A different type of temperature effect on the rate of ATP hydrolysis by the E. coli F1-ATPase was described by Laget over 25 years ago [17]. The rate of ATP hydrolysis as measured at 10° C was found to be dependent upon the incubation temperature of the enzyme prior to the assay. This was interpreted as a slow conversion between two conformational states of the enzyme, termed a high activity state and a low activity state. It was called a hysteretic property, according to the definition of Frieden [18, 19], in which such an enzyme responds slowly to a rapid change in ligand concentration. In this case, it is a slow response to a change in temperature, and so can be referred to as thermohysteresis.
Laget also examined the effects of ATP and ADP on the hysteretic properties of the E. coli F1-ATPase [20]. Pre-incubation of the enzyme with ATP or with ADP affected both the equilibrium between the two states, and the kinetic properties of the two states. Both nucleotides shifted the equilibrium towards the high activity state, but they had different effects on kinetic properties. The results were interpreted in terms of a high activity state that is stabilized by binding ATP, and a low activity state that is stabilized by binding ADP. The ratio of ATP/Mg2+ was kept constant at 2.0 during assays, and thus the role of Mg2+ was not investigated.
An intriguing feature of mitochondrial and chloroplast F1-ATPases is unidirectional inhibition by Mg-ADP in the direction of hydrolysis of ATP [21-24]. Hysteretic behavior with respect to ADP binding has been described in a variety of systems [20, 21, 25, 26]. Mg-ADP inhibition has been studied extensively in the F1-ATPase from the thermophilic Bacillus PS3 [27-29], but less so in the enzyme from E. coli [30, 31]. In the case of the bacterial F1-ATPase, studies of inhibition can be complicated by the epsilon subunit, an endogenous inhibitor that can partially dissociate during assay conditions [32, 33].
Recent studies in this laboratory have resulted in a rapid procedure for isolation of the ATP synthase from E. coli in a highly active form [34]. Procedures for the measurement of ATP hydrolysis and ATP-dependent proton translocation in continuous assay systems were optimized. During the course of these studies an unusual temperature dependence of ATP hydrolysis by the isolated F1Fo was observed, which resembled prior results obtained by Laget using F1-ATPase [15, 18]. Therefore, the sensitivity of F1Fo to low temperature was explored systematically, and its possible relationship to Mg-ADP inhibition was examined.
2. Materials and methods
2.1 Purificiation and preparations
F1Fo was purified from E. coli strain DK8 harboring plasmid pFV2, as previously described [34]. Growth of cultures, preparation of membrane vesicles, purification of F1Fo, and reconstitution into liposomes were also carried out as described previously [34]. Azide-treated membranes vesicles, termed N-particles, were prepared by two-fold dilution with medium containing 250 mM sucrose, 10 mM HEPES, pH 8.0, 5 mM MgCl2, 100 mM KCl, 0.1 mM EDTA, 2 mM malonate (potassium salt), 1 mg/ml bovine serum albumin, and 2 mM NaN3. Phosphate-treated membrane vesicles, termed P-particles, were prepared by two-fold dilution with medium containing 250 mM sucrose, 10 mM HEPES, pH 8.0, 10 mM phosphate (sodium salt), 100 mM KCl, 0.1 mM EDTA, 2 mM malonate, 1 mg/ml bovine serum albumin. Pretreatment was carried out for 1 hr at room temperature.
2.2 Functional assays.
ATP hydrolysis activity was measured either with an ATP regenerating system or with the pH-indicator phenol red, using the following conditions, unless otherwise specified. For measurements with an ATP regenerating system the medium (1 ml) contained 10 mM HEPES/KOH, pH 8.0, 100 mM KCl, 2.5 mM MgCl2, 0.1 mM EDTA, 1 mM ATP, 200 μM NADH, 2 mM phosphoenolpyruvate, lactate dehydrogenase (5 units/ml), pyruvate kinase (5 units/ml), and 5 μM FCCP (in the case of reconstituted F1Fo). Activity was measured by following the decrease in optical density at 340 nm. Using the pH-indicator phenol red, ATP hydrolysis activity was measured at 557 nm by following scalar proton release, essentially as described [34]. The medium (2 ml) contained 10 mM HEPES/KOH, pH 8.0, 100 mM KCl , 10 mM ATP, 4 mM MgCl2, 0.1 mM EDTA, 60 μM phenol red, and 5 μM FCCP (in the case of membrane vesicles). The phenol red assay was always checked at pH 8.0 to ensure that the ratio of protons released to phosphate released during ATP hydrolysis was approximately 1.0. Measurements of ATP-dependent ACMA-fluorescence quenching were performed as described previously [34]. In all assays, volumes of enzyme added were 5-20 μl per reaction. ATP synthesis by preparations of membrane vesicles was monitored using a modified PTI Delta-Scan 1 fluorimeter, which allowed real-time measurement of ATP synthesis using luciferase. Prior to measurement of ATP synthesis, membrane vesicles were resuspended in 1 ml of French press buffer (200 mM Tricine/HCl, pH 7.8, 100 mM KCl, 5 mM MgCl2, 0.1 mM EDTA, and 2.5% glycerol), passed through a 10-ml Sephadex column, equilibrated with the same buffer, ultracentrifuged as before, and resuspended in French press buffer. This step eliminated any energization-independent ATP synthase activity. The membranes were diluted twice with buffer used for preparation of P-particles. The reaction was initiated by addition of 20 mM succinate to 2 ml of a medium containing 200 μg membrane protein, 10 mM Tricine/KOH, pH 8.0, 100 mM KCl, 5 mM MgCl2, 1 mM Pi, 0.1 mM ADP, 125 μM luciferin, and 100 ng luciferase. 20 nmol ATP was added for calibration after each reaction was finished. No ATP synthesis was observed without ADP or Pi , or with uncoupler FCCP.
2.3 Analytical methods.
Native electrophoresis and determination of protein concentration were performed as described earlier [34].
3. Results
3.1 Temperature dependence of ATP hydrolysis.
Fig. 1A shows the time course of ATP hydrolysis by isolated E. coli F1Fo using an ATP-regenerating assay at 15°C. When enzyme was taken directly from ice (“cold” enzyme), its activity demonstrated a pronounced lag phase (trace b). Conversely, the activity of the enzyme after pre-incubation for 5 min at room temperature (“hot” enzyme) showed no lag phase, and was higher than in the first case (trace a). If the “hot” enzyme was returned to ice for 5 min before activity measurement, the time course became similar to that of the “cold” one (trace c). When the enzyme was subjected to additional cycles of warming and cooling, it exhibited the behavior of the “hot” or “cold” enzyme, according to its last incubation (data not shown). When the activity measurements were made at 37° C the duration of the lag phase for the “cold” enzyme (Fig. 1B, trace b) was much shorter, and there was no significant difference in the steady-state rates of the two forms of enzyme. In panel A the initial rate of the “cold” enzyme is shown by the straight line marked d. In five different experiments, the average ratio of the initial rates (traces d and a) was 0.53 ± .06 for isolated F1Fo.
Fig. 1.
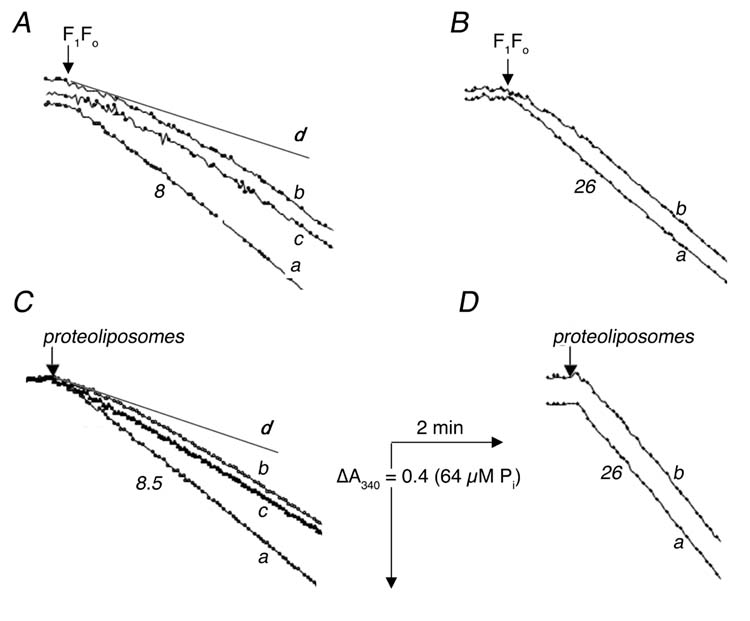
Time-course of ATP hydrolysis by isolated F1Fo (A, B) and by F1Fo incorporated into proteoliposomes (C, D) at 15° C (A, C) and at 37° C (B, D). ATPase activity was measured with an ATP regenerating system as described in “Experimental Procedures”. The reactions were initiated by addition of 2.5 μg (A), 0.7 μg (B), 1.2 μg (C) or 0.4 μg (D) of protein. In all panels trace a represents “hot” enzyme, in which the sample was taken from ice and pre-incubated at room temperature for 5 min before measurement. All traces b represent “cold” enzyme, in which the sample was taken directly from ice for measurement In panels (A) and (C) trace c represents enzyme that was taken from ice, then incubated at room temperature for 5 min, and returned to ice for 5 min, before measurement, and the lines marked d represent the initial rates of “cold” enzyme (trace b). Numbers shown near the curves indicate the rates of ATP hydrolysis expressed in μmol ATP per min per mg of protein.
The same set of experiments were conducted for the case of F1Fo reconstituted into liposomes (Fig. 1, CD). Similar patterns of activity were observed: a lag phase for the “cold” enzyme when assayed at 15° C with a reversible transition between the “hot” and “cold” enzyme (Fig. 1C), and no significant difference between the two forms of enzyme when assayed at 37° C (Fig. 1D). In panel C the initial rate of the “cold” enzyme is shown by the straight line marked d. In five different experiments, the average ratio of the initial rates (traces d and a) was 0.49 ± .06 for proteoliposomes. The two forms of enzyme were also tested for ATP-dependent proton translocation by the ACMA fluorescence quenching assay. At 15° C the initial rate of ATP-dependent ACMA fluorescence quenching by the “cold” enzyme, previously reconstituted into liposomes (Fig. 2A, trace b), was about one-half that of the “hot” enzyme (trace a). In eight experiments, the average ratio of initial rates was 0.54 ± .03. As before, the rate of quenching by the “hot” reconstituted enzyme, returned to ice for 5 min, was the same as that of the “cold” enzyme (trace c). There was no difference in the initial rate of quenching between the two forms of enzyme when the assay was carried out at 37° C (Fig. 2B). One difference between the ATP hydrolysis assay, shown in Fig. 1, and the proton translocation assay, shown in Fig. 2, is the length of time from addition of the enzyme until the start of recording. For technical reasons this interval is about 30 sec for ATP hydrolysis, and about 1 min for proton translocation, and this might affect the estimation of the initial rates.
Fig. 2.
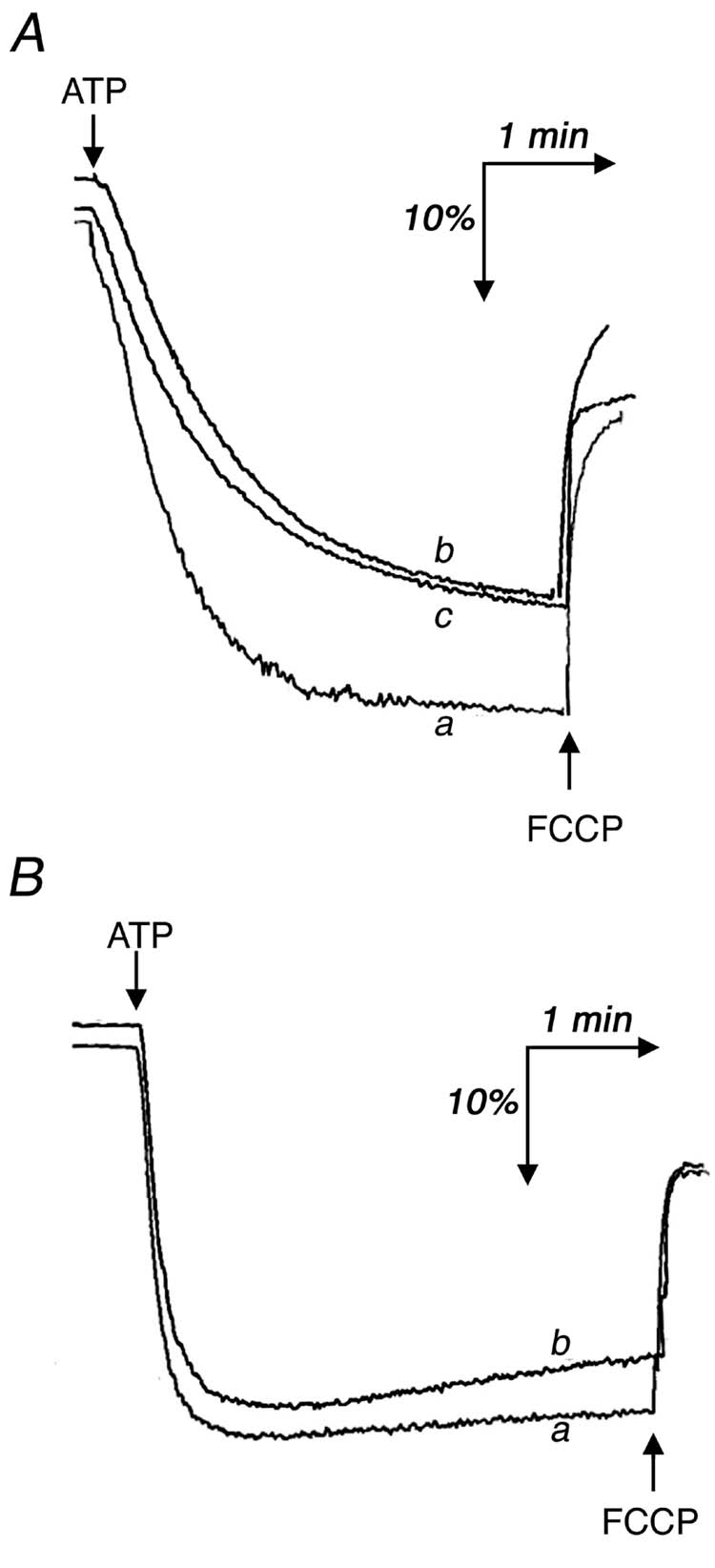
ATP-dependent ACMA fluorescence quenching by reconstituted F1Fo at 15° C (A) and at 37° C (B). The assay was carried out as described in “Experimental Procedures” using 1.2 μg of protein. The reaction was initiated by the addition of 0.2 mM ATP. In both panels trace a represents “hot” enzyme, in which the sample was taken from ice and pre-incubated at room temperature for 5 min before measurement; trace b represents “cold” enzyme, in which the sample was taken directly from ice for measurement. In panel (A) trace c represents enzyme that was taken from ice, then incubated at room temperature for 5 min, and returned to ice for 5 min, before measurement.
3.2 Dissociation of the enzyme.
As was first shown about forty years ago, soluble F1-ATPase promptly loses its activity at low temperature [15]. This cold inactivation is accompanied by dissociation of F1 into sub-complexes. In contrast, the thermohysteretic behavior of E. coli F1-ATPase as discovered by Laget [17], is not due to cold dissociation of the subunits. To check for the possibility of subunit dissociation by F1Fo, two approaches were used: estimation of ATPase activity after multiple cycles of temperature variation, and analysis of treated samples by native gel electrophoresis.
In the first approach, the “cold” enzyme was frozen at -20° C for 1 hr and subsequently thawed at 0° C for 40 min. The thawed enzyme was divided into two portions. One portion was incubated on ice, and the other was incubated at room temperature for 5 min before measurement of activity. The time course of ATP hydrolysis by these enzymes was similar to the unfrozen “hot” and “cold” ones. Recovery of the ATPase activity of the “hot” enzyme after freeze-thawing was 80% (data not shown).
In a second approach F1Fo was first pre-incubated either on ice or at room temperature for 5 min, and then frozen rapidly in liquid nitrogen (< 5 sec). The frozen suspensions were thawed at 37° C until reaching 0° C (about 5 sec) and immediately thereafter the ATPase activity was measured. Even this severe freeze-thaw cycle did not considerably change the time courses (or activities) of ATP hydrolysis observed, relative to the unfrozen “hot” and “cold” enzymes, shown previously.
Finally, the possibility of subunit dissociation was investigated directly by native gel electrophoresis. F1Fo was taken up either from ice or after a slow cycle of freezing-thawing and was run on native gels at 4° C, and at room temperature, for 50 min, as described previously [34]. At both temperatures only a single band was observed, indicating that F1Fo remained intact under all conditions and did not undergo cold dissociation (data not shown).
3.3 Investigations into the mechanism of thermohysteretic behavior.
F1-ATPases are well known to be inhibited generally by ADP, and the E. coli enzyme in particular is inhibited by Mg2+ ions [34, 35]. Furthermore, a Mg-ADP inhibited form of F1 has been identified in several different species, leading to hysteretic behavior [36, 37]. Azide is thought to stabilize the Mg-ADP inhibited form of F1, [31, 37, 38] although in E. coli this is controversial [30, 39]. The E. coli F1 is inhibited by azide, with a Ki of 25 μM at 37° C and 7 μM at 25° C [40]. Therefore, the relationship of Mg2+ ions, ADP and azide to the thermohysteretic properties described above was investigated.
In the previous experiments (Fig. 1), ATP hydrolysis was measured using an ATP regenerating system, in which little or no ADP accumulates during the assay. To investigate the consequences of the accumulation of ADP as ATP is hydrolyzed, a phenol red system was also used. The difference between the two assay systems can be demonstrated by comparing the rates of ATP hydrolysis by the isolated F1Fo ATP synthase, previously incubated for 5 min at room temperature (“hot” enzyme), at both 37° C and at 15° C. At 37° C the rate found in the phenol red assay system is 95% of that using the ATP regenerating system. At 15° C the rate in the phenol red system is only 31% of that in the ATP regenerating system. This suggests that the isolated F1Fo is especially sensitive to inhibition by ADP at low temperatures. Furthermore, in the presence of 100 μM azide, while the enzyme is seen to be inhibited using both assay systems, the rate is essentially zero using the phenol red assay, and is less than 5% of the rate in the ATP regenerating system.
In contrast to the isolated enzyme (Fig. 1), the ATPase activity of membrane vesicles did not depend upon the temperature of pre-incubation when measured at 15° C as shown in Fig.3A. In trace b the membranes were taken directly from ice, and in trace a, the membrane were pre-incubated for 5 min at room temperature. There was also no effect of pre-incubation when assayed at 37° C (data not shown). These measurements were made using the phenol red assay, to avoid complications due to NADH dehydrogenases present in the membranes. For comparison, the results from isolated F1Fo assayed under the same conditions of Fig. 3A are shown in panel B, in which a lag phase for the “cold” enzyme is apparent. Pre-incubation of the enzyme (isolated, reconstituted F1Fo, or in membrane vesicles) on ice for more than 5 min did not change the pattern of activities seen in Figs. 1, 2, or 3. The traces shown are typical ones from several experiments.
Fig. 3.
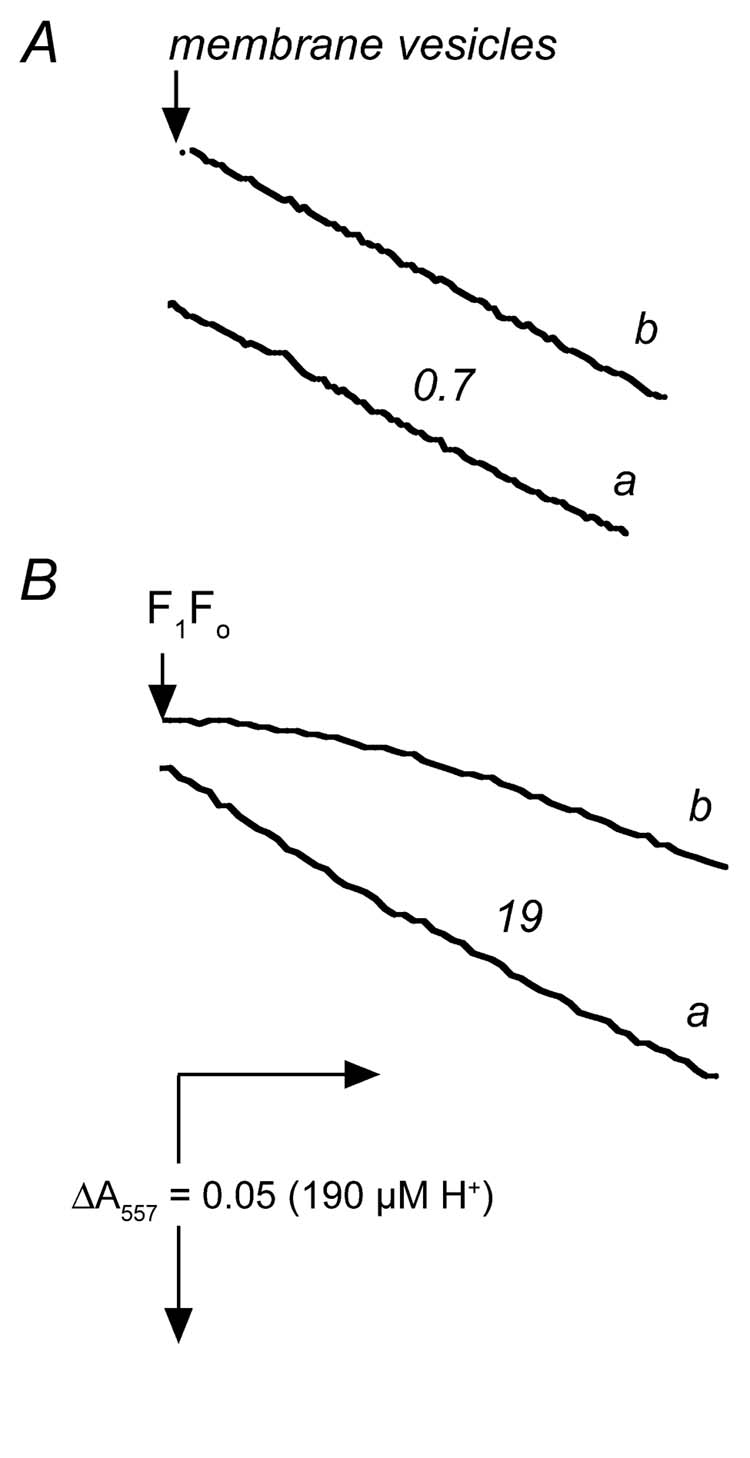
Time-courses of ATP hydrolysis by membrane vesicles (A) and by isolated F1Fo (B) without an ATP-regenerating assay system. In both panels ATPase activity was measured with phenol red at 15° C as described in “Experimental Procedures”, using 220 μg (A) or 7 μg (B) of protein. In both panels trace a represents “hot” enzyme, in which the sample was taken from ice and pre-incubated at room temperature for 5 min before measurement; trace b represents “cold” enzyme, in which the sample was taken directly from ice for measurement. Numbers shown near the curves indicate the rates of ATP hydrolysis expressed in μmol ATP per min per mg of protein.
The addition of up to 200 μM ADP to the pre-incubation medium had no effect on ATPase activity under all conditions tested. In contrast, pre-incubation with 1 mM azide, even in the absence of exogenous ADP, had profound effects on the initial rates of ATPase activity, as shown by the following experiments. Membrane vesicles that were pre-incubated with 1 mM azide for 1 hr at room temperature (called N-particles) were compared with those that were pre-incubated without azide (control). The results, as shown in Table I, indicate that azide is a potent inhibitor of the initial rate of ATPase activity, in a way that depends upon both temperature and the concentrations of ATP and Mg2+. When assayed at 10° C, membrane vesicles had initial rates of ATP hydrolysis that varied with the ATP concentration (Mg2+ was kept at 4 mM). Under the same assay conditions, membranes pre-incubated with azide (N-particles) had initial rates of zero. After 7 min, the rates increased somewhat, if assayed at higher concentrations of ATP (Table I and Fig. 4). If the assay medium also included 10 mM Pi, the lag phase was extended, and the rate decreased, as shown in Fig. 4 (comparing traces b and c). Inhibition of initial rates due to pre-incubation with azide was completely eliminated if the assay medium included 0.3% LDAO (Table I, assay 4). Under assay conditions of 10 mM ATP/4 mM Mg2+, if the assay temperature was increased from 10° C (assay 3) to 25° C (assay 5) or to 37° C (assay 6) the initial rates increased dramatically at the higher temperatures. In addition, the effect of pre-incubation with azide decreased from 100% inhibition at 10° C to only 6% at 37° C.
Table 1.
Inhibition of ATP hydrolysis by pre-incubation with azide at 23°Ca
| Assay Number | Assay Conditionsb (mM ATP/mM Mg2+) | Initial rates of ATP hydrolysis (μmol/min/mg protein) | Ratio of rates (N-particles/Control) | Rates of ATP hydrolysis by N-particles after 7 min in assay medium | |
|---|---|---|---|---|---|
| Control | N-particles | ||||
| 1 | 10° C (0.5/4) | 0.16 | 0 | 0 | 0 |
| 2 | 10° C (2/4) | 0.27 | 0 | 0 | 0.045 |
| 3 | 10° C (10/4) | 0.31 | 0 | 0 | 0.12 |
| 3 | 10° C (10/4) | 0.31 | 0 | 0 | 0.12 |
| 4 | 10° C (0.5/4) 0.3% LDAO | 0.2 | 0.2 | 1.0 | NDc |
| 5 | 25° C (10/4) | 0.75 | 0.56 | 0.74 | ND |
| 6 | 37° C (10/4) | 1.54 | 1.44 | 0.94 | ND |
Rates shown are a typical set of values from at least three different experiments with similar results. N-particles were prepared by two-fold dilution of membrane vesicles with medium containing 250 mM sucrose, 10 mM HEPES, pH 8.0, 5 mM MgCl2, 100 mM KCl, 0.1 mM EDTA, 2 mM malonate (potassium salt), 2 mM NaN3, and incbated at room temperature for 1 h. Control particles were treated similarly, without the NaN3.
Reactions were initiated by addition of 415 μg membrane protein (up to 20 μl) to 2 ml of medium, using the phenol red system.
ND, Not determined
Fig. 4.
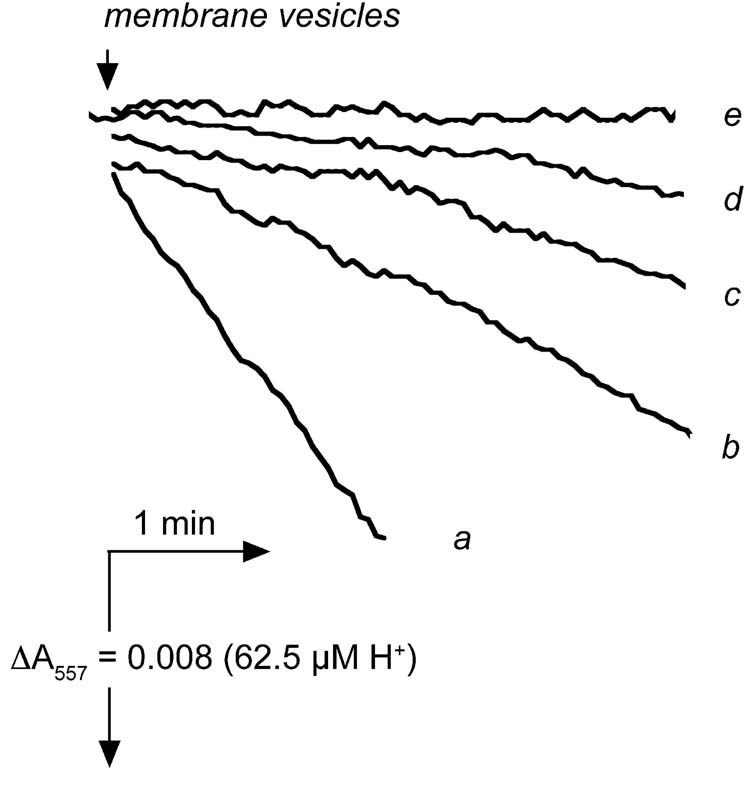
Time courses of ATP hydrolysis by membrane vesicles pre-incubated with azide. Rates of ATP hydrolysis were measured using the phenol red assay system at 10° C in the presence of 4 mM Mg2+. Trace a shows the rate of control membrane vesicles using 10 mM ATP. Traces b-e are from azide-treated membranes (N-particles). Trace b shows the rate of ATP hydrolysis by N-particle in the presence of 10 mM ATP. Trace c is the same as trace b, except that the medium contains 10 mM Pi. Trace d shows the rate of ATP hydrolysis by N-particles using 2 mM ATP, and trace e shows the rate with 0.5 mM ATP. All samples were kept at room temperature prior to the assay. The traces shown were typical ones from at least three sets of measurements.
The effects of azide seen here are due primarily to the pre-incubation, since if the equivalent amount of azide is diluted into the assay medium the inhibition is only about 7%, if measured at 10° C. Furthermore, the effects of azide are limited to ATP hydrolysis, since pre-incubation of membrane vesicles with azide had no effect on the rates of ATP synthesis when assayed at 5° C to 22° C. For example, at 10° C the measured rates of ATP synthesis were between 11 and 13 nmol ATP/min/mg protein for N-particles and control particles, with or without a pre-incubation with 2 mM Pi. No lag phase was apparent at any temperature. Likewise, the rates at 22° C were between 28 and 30 nmol ATP/min/mg protein in each case.
The effect of Mg2+ concentration on the thermohysteretic properties of F1Fo was also investigated, and the results are presented in Fig. 5. The ATP concentration was fixed at 10 mM and the Mg2+ concentration was varied from 1 to 20 mM. Rates of ATP hydrolysis by isolated F1Fo (panel A) and by membrane vesicles (panel B) were measured using the phenol red method. Samples were pre-incubated for 5 min at room temperature, and assayed at 15° C (filled circles) or 37° C (open circles). The rates of ATP hydrolysis by isolated F1Fo at both temperatures show a similar dependence upon Mg2+ concentration (A), but by plotting the ratio of the rate at 15° C over the rate at 37° C it can be seen that ATP hydrolysis by isolated F1Fo at 15° C is increasingly inhibited by Mg2+ (panel C, squares). This indicates that Mg2+ is an essential component of the thermohysteretic properties of the “cold” enzyme. In contrast, the ratio of the rates of ATP hydrolysis by membrane vesicles at the two temperatures does not depend upon Mg2+ concentration (panel 6C, triangles). This is consistent with the prior observation (Fig. 4) that F1Fo in membrane vesicles does not exhibit thermohysteretic properties. A plot of the ratio of rates at the two temperatures by F1Fo reconstituted into liposomes falls between the other two plots (data not shown).
Fig. 5.
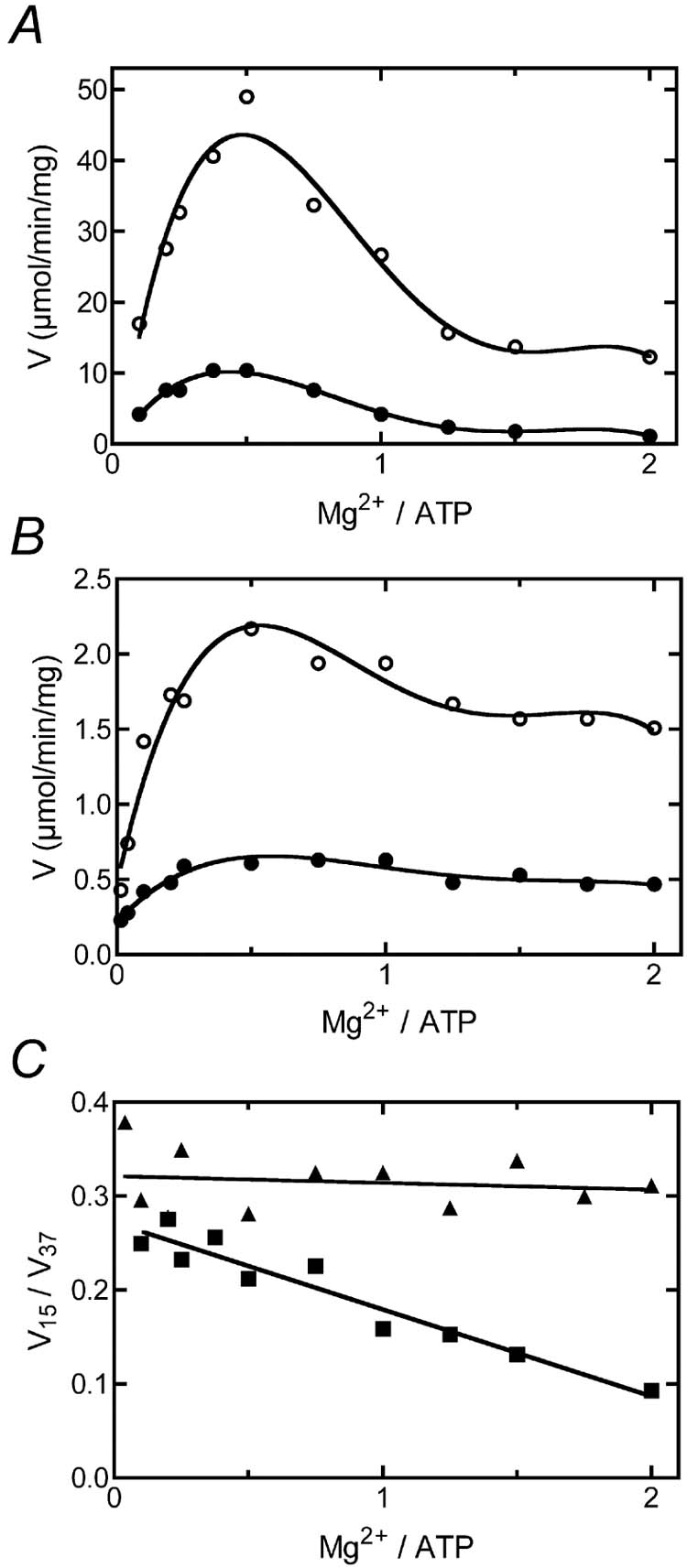
The effect of Mg2+ concentration on the thermohysteretic properties of F1Fo. In panel A, the rates of ATP hydrolysis by “hot” isolated F1Fo were measured using the phenol red system at 37° C (open circles) and at 15° C (filled circles). In panel B the same measurements were made using membrane vesicles at 37° C (open circles) and at 15° C (filled circles). In panel C the ratios of the rates at 15° and at 37° are plotted for the isolated F1Fo (squares) and for membrane vesicles (triangles).
4. Discussion
The thermohysteretic behavior described previously by Laget [17, 20] for hydrolysis of ATP by the soluble F1-ATPase from E. coli has been shown to occur in the isolated F1Fo ATP synthase. Laget showed that the rate of ATP hydrolysis at 10° C depends upon the temperature of pre-incubation of the enzyme. He found that F1-ATPase incubated at low temperature had lower rates than did identical samples pre-incubated at higher temperatures. We found a similar dependence upon the rate of ATP hydrolysis by the isolated F1Fo ATP synthase when measured at 15° C. These results appear to extend to both ATP hydrolysis and ATP-driven proton translocation by the isolated enzyme, reconstituted in liposomes. Remarkably, ATP hydrolysis by membrane vesicles at 15° C did not exhibit a lag phase when pre-incubated on ice, suggesting that the transition was too rapid to be observed. This might be a consequence of the native lipid environment, but further studies will be necessary to understand this.
Hysteretic behavior was not a consequence of the well-known cold denaturation of F1-ATPase [14-16]. Native gel electrophoresis showed that subunit dissociation did not occur as a result of the incubation on ice, and multiple cycles of pre-incubation temperatures showed that this transition was readily reversible. As was concluded by Laget [17, 20] with respect to F1, this behavior is consistent with two states of the enzyme in equilibrium: a high activity state and a low activity state. At temperatures above 25° C, the states are in rapid equilibrium, and the high activity state is favored. At temperatures below 15° C, the low activity state becomes more prevalent, and the rate of inter-conversion between the two states is reduced.
Further experiments showed that the low temperature state of the F1Fo ATP synthase is more sensitive to inhibition by the presence of ADP or Mg2+ in the assay medium, and to azide in the pre-incubation medium. Therefore, it is proposed that the low activity state of the isolated F1Fo can be stabilized by tightly-bound Mg-ADP. Both mitochondrial and chloroplast F1-ATPases are thought to be subject to inhibition by tightly-bound Mg-ADP [21, 26, 37, 38], leading to hysteretic behavior. This inhibition is gradually lost after the addition of sufficient ATP, such as occurs during an assay of ATP hydrolysis, and can be seen as a lag phase in ATP hydrolysis. The F1-ATPase from the thermophilic Bacillus PS3 has been extensively studied with respect to such inhibition [28, 29, 41]. For example, the loss of ATP binding due to mutations at non-catalytic sites was shown to greatly enhance the inhibition of ATP hydrolysis by tightly-bound Mg-ADP. LDAO was found to eliminate Mg-ADP inhibition when included in the assay medium at a concentration of 0.1%, and similar results were shown here in Table I.
Prior studies of the E. coli F1-ATPase have failed to detect Mg-ADP inhibition of ATP hydrolysis under assay conditions at room temperature or above [30]. Hyndman et al [31] demonstrated a lag phase in ATP hydrolysis by incubating the F1-ATPase, free of its epsilon subunit, in Mg-ADP, then diluting into 50 nM ATP, 50 μM Mg2+. Since the studies reported here used intact F1Fo, there were no complications from dissociation of the inhibitory epsilon subunit that can occur by dilution of the F1-ATPase during the assay. The low temperatures used in this study appear to have been essential for observation of the lag phase in ATP hydrolysis by isolated F1Fo.
The apparent initial rate of ATP hydrolysis depends primarily upon temperature, but is also influenced by the method of assay. As shown in Fig. 3, the thermohysteretic effect is enhanced when using an assay that does not regenerate ATP. It appears that at low temperatures inhibitory ADP binds with high affinity to the isolated F1Fo. This ADP could be from commonly found endogenous ADP [30, 42], from ATP hydrolysis, or from ADP contamination of commercial ATP. Recent results by Turina et al. [43] showed that at 34° C, the rate of ATP hydrolysis as measured by the phenol red system were virtually identical to the rates measured by an ATP regenerating system, using membrane vesicles from Rhodobacter capsulatus. At 23° C, the rate measured by phenol red was only about 70% of the maximal rate measured using a regenerating system, and it exhibited a slight lag phase. Those authors did not invoke temperature as an explanation of the difference in rates, but our results would support such an interpretation.
Results presented in Fig. 4 suggest that inorganic phosphate might play a similar inhibitory role to azide at low temperature. Fischer et al [44] showed that ATP hydrolysis by the E. coli F1Fo ATP synthase in liposomes was inhibited sharply by a combination of inorganic phosphate and ADP in the assay medium. Our results indicate that such inhibition is enhanced at low temperature, and when using a phenol red assay. The essential role of Mg2+ in the thermohysteretic properties of F1Fo is also demonstrated by results presented here. The inhibition of ATP hydrolysis by the E. coli enzyme due to high concentrations of Mg2+ is well-known [34, 35]. The optimal ratio of Mg2+/ATP for ATP hydrolysis is approximately 0.3-0.5, and at higher concentrations Mg2+ is inhibitory. Our results show that the response to Mg2+ of ATP hydrolysis by isolated F1Fo is temperature dependent. Above the optimal concentration of Mg , the isolated enzyme is increasingly inhibited at 15° C relative to at 37° C, as shown in Fig. 5C.
Inhibition of the mitochondrial and chloroplast F1-ATPase by azide is thought to occur by stabilization of the Mg-ADP inhibited state. Hyndman et al [31] have shown a correlation between inhibition of ATP hydrolysis of the E. coli F1-ATPase by azide, and the amount of tightly bound ADP present after passage through a centrifuge column. Their results were seemingly contradicted by studies [39] that showed that 3 molecules of ATP are bound at catalytic sites in the presence of inhibitory azide, by monitoring the fluorescence of reporter tryptophan residues at the catalytic sites. Those results show that ADP binding at catalytic sites is not necessary for inhibition by azide, although ATP binding did occur. Other studies, using the Bacillus PS3 enzyme have shown that it may not always be possible to distinguish between the binding of ADP and ATP [45] through such fluorescence measurements. Results presented here show that at low temperatures, and in the presence of Mg-ADP, azide is a potent inhibitor of ATP hydrolysis, but not of ATP synthesis.
The epsilon and gamma subunits are likely to play a role in the conformation of the low activity state, and in the binding of inhibitory Mg-ADP. For example, Yoshida and colleagues [10, 46] have discovered a partially-rotated state of the gamma subunit during assays of ATP hydrolysis using video microscopy. Gamma appears to pause after a rotation of about 85°, before it continues to the fully rotated position of 120°. It has been previously been suggested that the position of gamma is related to the binding of inhibitory Mg-ADP [47]. The position of the C-terminal alpha-helices of the epsilon subunit have also been implicated in the inhibition of ATP hydrolysis, in a nucleotide and inorganic phosphate dependent way [48, 49]. Recently Yoshida and colleagues [50] have shown that the C-terminus of epsilon and the N-terminus of gamma can form a cross-link by a disulfide bond during conditions that promote ATP synthesis, but not during ATP hydrolysis. LDAO is known to increase the rate of ATP hydrolysis by the E. coli F1 and F1Fo, and also to prevent a cross-link between the C-terminus of the epsilon and beta subunits [51, 52]. We propose that the low activity form of the E. coli F1Fo seen at low temperatures represents a low energy state, in which the gamma subunit is at an intermediate position, and the C-terminal alpha helix of the epsilon subunit is docked near the N-terminus of gamma. At sufficiently low temperatures the enzyme can entrap inhibitory Mg-ADP and show hysteretic behavior when assayed. This might be related to strengthening electrostatic interactions between the C-terminal alpha-helix of epsilon and the DELSEED region of the beta subunit. At higher temperatures, the enzyme more easily escapes from the low activity state. Azide is proposed to stablize the low activity state, increasing the affinity of Mg-ADP.
Studies by Fischer et al [44] have shown the importance of in stimulating the rate of ATP hydrolysis by the E. coli F1Fo reconstituted into liposomes and measured at 23° C. Activation was seen only if ADP or inorganic phosphate, which were inhibitory, were included in the assay. In the absence of ADP and inorganic phosphate the rate of ATP hydrolysis was equal to the activated rate. It can be imagined that this transient energization is able to rotate the gamma subunit in the direction of ATP synthesis, allowing escape from the deactivated state. Lack of a lag phase in ATP synthesis supports this view.
The results presented here also have important implications for interpretations of the temperature dependence of rates of ATP hydrolysis by the E. coli ATP synthase. Cold temperature deactivation of an enzyme could lead to breaks in an Arrhenius plot, as has been discussed by Silvius et al. [53, 54]. Membrane-dependent effects on the temperature dependence of ATP hydrolysis have been reported with the E. coli enzyme [55]. Other studies [56], using the bovine F1-ATPase have demonstrated breaks in the Arrhenius plot of ATP hydrolysis without a membrane environment. The activation energy at temperatures below the transition temperature, about 18° C, was diminished if the enzyme was depleted of endogenous nucleotides. Their results were entirely consistent with our findings that the E. coli ATP synthase undergoes cold deactivation that depends upon Mg2+ and ADP.
Acknowledgments
This work was supported by grant GM40508 from the National Institutes of Health, and grant N-1378 from the Welch Foundation. We thank Dr. Pia Vogel for helpful discussions, Dr. Larry Ruben for assistance in modifying the PTI fluorimeter for luminescence, and Dr. Ivan Charamisinau and the machine shop of the Department of Mechanical Engineering at SMU for assistance in construction of the luminometer cell chamber.
Footnotes
- DCCD
- N,´-dicyclohexylcarbodiimide
- FCCP
- carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone
- ACMA
- 9-amino-6-chloro-2-methoxyacridine
- LDAO
- lauryldimethylamine oxide.
References
- [1].Boyer PD. The ATP synthase - A splendid molecular machine. Ann. Rev. Biochem. 1997;66:717–749. doi: 10.1146/annurev.biochem.66.1.717. [DOI] [PubMed] [Google Scholar]
- [2].Bragg PD, Hou C. Subunit composition, function, and spatial arrangement in the Ca2+-and Mg2+-activated adenosine triphosphatases of Escherichia coli and Salmonella typhimurium. Arch. Biochem. Biophys. 1975;167:311–21. doi: 10.1016/0003-9861(75)90467-1. [DOI] [PubMed] [Google Scholar]
- [3].Foster DL, Fillingame RH. Stoichiometry of subunits in the H-ATPase complex of Escherichia coli. J. Biol. Chem. 1982;257:2009–15. [PubMed] [Google Scholar]
- [4].Jiang W, Hermolin J, Fillingame RH. The preferred stoichiometry of c subunits in the rotary motor sector of Escherichia coli ATP synthase is 10. Proc. Natl. Acad. Sci. U. S. A. 2001;98:4966–71. doi: 10.1073/pnas.081424898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Duncan TM, Bulygin VV, Zhou Y, Hutcheon ML, Cross RL. Rotation of subunits during catalysis by Escherichia coli F1-ATPase. Proc. Natl. Acad. Sci.. U. S. A. 1995;92:10964–8. doi: 10.1073/pnas.92.24.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sabbert D, Engelbrecht S, Junge W. Intersubunit rotation in active F- ATPase. Nature. 1996;381:623–5. doi: 10.1038/381623a0. [DOI] [PubMed] [Google Scholar]
- [7].Sambongi Y, Iko Y, Tanabe M, Omote H, Iwamoto-Kihara A, Ueda I, Yanagida T, Wada Y, Futai M. Mechanical rotation of the c subunit oligomer in ATP synthase (FF1): direct observation. Science. 1999;286:1722–4. doi: 10.1126/science.286.5445.1722. [DOI] [PubMed] [Google Scholar]
- [8].Itoh H, Takahashi A, Adachi K, Noji H, Yasuda R, Yoshida M, Kinosita K. Mechanically driven ATP synthesis by F1-ATPase. Nature. 2004;427:465–8. doi: 10.1038/nature02212. [DOI] [PubMed] [Google Scholar]
- [9].Noji H, Yasuda R, Yoshida M, Kinosita K. Direct observation of the rotation of F1-ATPase. Nature. 1997;386:299–302. doi: 10.1038/386299a0. [DOI] [PubMed] [Google Scholar]
- [10].Yasuda R, Noji H, Yoshida M, Kinosita K, Itoh H. Resolution of distinct rotational substeps by submillisecond kinetic analysis of F1-ATPase. Nature. 2001;410:898–904. doi: 10.1038/35073513. [DOI] [PubMed] [Google Scholar]
- [11].Hong S, Ko YH, Pedersen PL. Rotary catalysis within ATP synthases: a bioinformatic approach provides novel insight into how large pH-dependent movements of the C-terminal helix of subunit c may be accommodated. Arch. Biochem. Biophys. 2001;394:275–9. doi: 10.1006/abbi.2001.2549. [DOI] [PubMed] [Google Scholar]
- [12].Xing J, Wang H, von Ballmoos C, Dimroth P, Oster G. Torque generation by the Fo motor of the sodium ATPase. Biophys. J. 2004;87:2148–63. doi: 10.1529/biophysj.104.042093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Meier T, Polzer P, Diederichs K, Welte W, Dimroth P. Structure of the rotor ring of F-Type Na+-ATPase from Ilyobacter tartaricus. Science. 2005;308:659–62. doi: 10.1126/science.1111199. [DOI] [PubMed] [Google Scholar]
- [14].Bennun A, Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. IV. Interaction of coupling factor 1 from chloroplasts with components of the chloroplast membrane. J. Biol. Chem. 1969;244:1325–31. [PubMed] [Google Scholar]
- [15].Penefsky HS, Warner RC. Partial resolution of the enzymes catalyzing oxidative phosphorylation. VI. Studies on the mechanism of cold inactivation of mitochondrial adenosine triphosphatase. J. Biol. Chem. 1965;240:4694–702. [PubMed] [Google Scholar]
- [16].Vogel G, Steinhart R. ATPase of Escherichia coli: purification, dissociation, and reconstitution of the active complex from the isolated subunits. Biochemistry. 1976;15:208–16. doi: 10.1021/bi00646a032. [DOI] [PubMed] [Google Scholar]
- [17].Laget PP. Hysteretic properties of soluble F1 ATPase from Escherichia coli. Evidence for a slow transition between two conformational states of the enzyme. Arch. Biochem. Biophys. 1978;189:122–31. doi: 10.1016/0003-9861(78)90124-8. [DOI] [PubMed] [Google Scholar]
- [18].Frieden C. Kinetic aspects of regulation of metabolic processes. The hysteretic enzyme concept. J. Biol. Chem. 1970;245:5788–99. [PubMed] [Google Scholar]
- [19].Frieden C. Slow transitions and hysteretic behavior in enzymes. Annu. Rev. Biochem. 1979;48:471–89. doi: 10.1146/annurev.bi.48.070179.002351. [DOI] [PubMed] [Google Scholar]
- [20].Laget PP. Hysteretic properties of soluble F1 ATPase from Escherichia coli. II. Nucleotide effects on the slow changes of the enzyme kinetic behaviour. Arch. Biochem. Biophys. 1979;192:474–81. doi: 10.1016/0003-9861(79)90117-6. [DOI] [PubMed] [Google Scholar]
- [21].Jault JM, Allison WS. Hysteretic inhibition of the bovine heart mitochondrial F1-ATPase is due to saturation of noncatalytic sites with ADP which blocks activation of the enzyme by ATP. J. Biol. Chem. 1994;269:319–25. [PubMed] [Google Scholar]
- [22].Fitin AF, Vasilyeva EA, Vinogradov AD. An inhibitory high affinity binding site for ADP in the oligomycin-sensitive ATPase of beef heart submitochondrial particles. Biochem. Biophys. Res. Commun. 1979;86:434–9. doi: 10.1016/0006-291x(79)90884-2. [DOI] [PubMed] [Google Scholar]
- [23].Shoshan V, Strotmann H. The effect of phosphate on light-induced exchange of ADP at the tight nucleotide binding site of CF1. J. Biol. Chem. 1980;255:996–9. [PubMed] [Google Scholar]
- [24].Recktenwald D, Hess B. A slow conformation change in the transient state kinetics of soluble ATPase of yeast mitochondria. FEBS Lett. 1977;80:187–9. doi: 10.1016/0014-5793(77)80436-5. [DOI] [PubMed] [Google Scholar]
- [25].Schobert B, Lanyi JK. Hysteretic behavior of an ATPase from the archaebacterium, Halobacterium saccharovorum. J. Biol. Chem. 1989;264:12805–12. [PubMed] [Google Scholar]
- [26].Di Pietro A, Penin F, Godinot C, Gautheron DC. "Hysteric" behavior and nucleotide binding sites of pig heart mitochondrial F1 adenosine 5'- triphosphatase. Biochemistry. 1980;19:5671–8. doi: 10.1021/bi00566a002. [DOI] [PubMed] [Google Scholar]
- [27].Paik SR, Jault JM, Allison WS. Inhibition and inactivation of the F1 adenosinetriphosphatase from Bacillus PS3 by dequalinium and activation of the enzyme by lauryl dimethylamine oxide. Biochemistry. 1994;33:126–33. doi: 10.1021/bi00167a016. [DOI] [PubMed] [Google Scholar]
- [28].Jault JM, Matsui T, Jault FM, Kaibara C, Muneyuki E, Yoshida M, Kagawa Y, Allison WS. The αβ γ complex of the F1-ATPase from thermophilic Bacillus PS3 containing the α D261N substitution fails to dissociate inhibitory MgADP from a catalytic site when ATP binds to noncatalytic sites. Biochemistry. 1995;34:16412–8. doi: 10.1021/bi00050a023. [DOI] [PubMed] [Google Scholar]
- [29].Jault JM, Dou C, Grodsky NB, Matsui T, Yoshida M, Allison WS. The αβγ subcomplex of the F1-ATPase from the thermophilic Bacillus PS3 with the βT165S substitution does not entrap inhibitory MgADP in a catalytic site during turnover. J. Biol. Chem. 1996;271:28818–24. doi: 10.1074/jbc.271.46.28818. [DOI] [PubMed] [Google Scholar]
- [30].Senior AE, Lee RS, al-Shawi MK, Weber J. Catalytic properties of Escherichia coli F1-ATPase depleted of endogenous nucleotides. Arch. Biochem. Biophys. 1992;297:340–4. doi: 10.1016/0003-9861(92)90682-m. [DOI] [PubMed] [Google Scholar]
- [31].Hyndman DJ, Milgrom YM, Bramhall EA, Cross RL. Nucleotide- binding sites on Escherichia coli F1-ATPase. Specificity of noncatalytic sites and inhibition at catalytic sites by MgADP. J. Biol. Chem. 1994;269:28871–7. [PubMed] [Google Scholar]
- [32].Smith JB, Sternweiss PC. Purification of membrane attachment and inhibitory subunits of the proton translocating adenosine triphosphatase from Escherichia coli. Biochemistry. 1977;16:306–311. doi: 10.1021/bi00621a023. [DOI] [PubMed] [Google Scholar]
- [33].Sternweiss PC, Smith JB. Characterization of the inhibitory (ε) subunit of the proton-translocating adenosine triphosphatase from Escherichia coli. Biochemistry. 1980;19:526–531. doi: 10.1021/bi00544a021. [DOI] [PubMed] [Google Scholar]
- [34].Ishmukhametov RR, Galkin MA, Vik SB. Ultrafast purification and reconstitution of His-tagged cysteine-less Escherichia coli F1Fo ATP synthase. Biochim. Biophys. Acta. 2005;1706:110–6. doi: 10.1016/j.bbabio.2004.09.012. [DOI] [PubMed] [Google Scholar]
- [35].Wise JG, Duncan TM, Latchney LR, Cox DN, Senior AE. Properties of F1-ATPase from the uncD412 mutant of Escherichia coli. Biochem. J. 1983;215:343–50. doi: 10.1042/bj2150343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Milgrom YM, Ehler LL, Boyer PD. The characteristics and effect on catalysis of nucleotide binding to noncatalytic sites of chloroplast F1-ATPase. J. Biol. Chem. 1991;266:11551–8. [PubMed] [Google Scholar]
- [37].Murataliev MB, Milgrom YM, Boyer PD. Characteristics of the combination of inhibitory Mg2+ and azide with the F1 ATPase from chloroplasts. Biochemistry. 1991;30:8305–10. doi: 10.1021/bi00098a004. [DOI] [PubMed] [Google Scholar]
- [38].Vasilyeva EA, Minkov IB, Fitin AF, Vinogradov AD. Kinetic mechanism of mitochondrial adenosine triphosphatase. Inhibition by azide and activation by sulphite. Biochem. J. 1982;202:15–23. doi: 10.1042/bj2020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Weber J, Senior AE. Effects of the inhibitors azide, dicyclohexylcarbodiimide, and aurovertin on nucleotide binding to the three F1 ATPase catalytic sites measured using specific tryptophan probes. J. Biol. Chem. 1998;273:33210–5. doi: 10.1074/jbc.273.50.33210. [DOI] [PubMed] [Google Scholar]
- [40].Noumi T, Maeda M, Futai M. Mode of inhibition of sodium azide on H- ATPase of Escherichia coli. FEBS Lett. 1987;213:381–4. doi: 10.1016/0014-5793(87)81526-0. [DOI] [PubMed] [Google Scholar]
- [41].Bald D, Muneyuki E, Amano T, Kruip J, Hisabori T, Yoshida M. The noncatalytic site-deficient αβγ subcomplex and FoF1-ATP synthase can continuously catalyse ATP hydrolysis when P is present. Eur. J. Biochem. 1999;262:563–8. doi: 10.1046/j.1432-1327.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- [42].Hanada H, Noumi T, Maeda M, Futai M. Uni-site catalysis by Escherichia coli F1-ATPase with different numbers of bound nucleotides. FEBS Lett. 1989;257:465–7. doi: 10.1016/0014-5793(89)81597-2. [DOI] [PubMed] [Google Scholar]
- [43].Turina P, Giovannini D, Gubellini F, Melandri BA. Physiological ligands ADP and P modulate the degree of intrinsic coupling in the ATP synthase of the photosynthetic bacterium Rhodobacter capsulatus. Biochemistry. 2004;43:11126–34. doi: 10.1021/bi048975+. [DOI] [PubMed] [Google Scholar]
- [44].Fischer S, Gräber P, Turina P. The activity of the ATP synthase from Escherichia coli is regulated by the transmembrane proton motive force. J. Biol. Chem. 2000;275:30157–62. doi: 10.1074/jbc.275.39.30157. [DOI] [PubMed] [Google Scholar]
- [45].Dong K, Ren H, Allison WS. The fluorescence spectrum of the introduced tryptophans in the α(βF155W)γ subcomplex of the F1-ATPase from the thermophilic Bacillus PS3 cannot be used to distinguish between the number of nucleoside di- and triphosphates bound to catalytic sites. J. Biol. Chem. 2002;277:9540–7. doi: 10.1074/jbc.M106911200. [DOI] [PubMed] [Google Scholar]
- [46].Yasuda R, Masaike T, Adachi K, Noji H, Itoh H, Kinosita K. The ATP-waiting conformation of rotating F1-ATPase revealed by single-pair fluorescence resonance energy transfer. Proc. Natl. Acad. Sci. U. S. A. 2003;100:9314–8. doi: 10.1073/pnas.1637860100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Allison WS, Jault JM, Dou C, Grodsky NB. Does the gamma subunit move to an abortive position of ATP hydrolysis when the F1.ADP.Mg complex isomerizes to the inactive F1*.ADP.Mg complex? J. Bioenerg. Biomembr. 1996;28:433–8. doi: 10.1007/BF02113985. [DOI] [PubMed] [Google Scholar]
- [48].Mendel-Hartvig J, Capaldi RA. Catalytic site nucleotide and inorganic phosphate dependence of the conformation of the epsilon subunit in Escherichia coli adenosinetriphosphatase. Biochemistry. 1991;30:1278–84. doi: 10.1021/bi00219a017. [DOI] [PubMed] [Google Scholar]
- [49].Mendel-Hartvig J, Capaldi RA. Nucleotide-dependent and dicyclohexylcarbodiimide-sensitive conformational changes in the epsilon subunit of Escherichia coli ATP synthase. Biochemistry. 1991;30:10987–91. doi: 10.1021/bi00109a025. [DOI] [PubMed] [Google Scholar]
- [50].Suzuki T, Murakami T, Iino R, Suzuki J, Ono S, Shirakihara Y, Yoshida M. FoF1-ATPase/synthase is geared to the synthesis mode by conformational rearrangement of epsilon subunit in response to proton motive force and ADP/ATP balance. J. Biol. Chem. 2003;278:46840–6. doi: 10.1074/jbc.M307165200. [DOI] [PubMed] [Google Scholar]
- [51].Lötscher HR, deJong C, Capaldi RA. Inhibition of the adenosinetriphosphatase activity of Escherichia coli F1 by the water-soluble carbodiimide 1-ethyl-3-[3-dimethylamino)propyl] carbodiimide is due to modification of several carboxyls in the β subunit. Biochemistry. 1984;23:4134–4140. doi: 10.1021/bi00313a019. [DOI] [PubMed] [Google Scholar]
- [52].Lötscher HR, deJong C, Capaldi RA. Interconversion of high and low adenosinetriphosphatase activity forms of Escherichia coli F1 by the detergent lauryldimethylamine oxide. Biochemistry. 1984;23:4140–4143. doi: 10.1021/bi00313a020. [DOI] [PubMed] [Google Scholar]
- [53].Silvius JR, McElhaney RN. Non-linear Arrhenius plots and the analysis of reaction and motional rates in biological membranes. J Theor Biol. 1981;88:135–52. doi: 10.1016/0022-5193(81)90332-5. [DOI] [PubMed] [Google Scholar]
- [54].Silvius JR, Read BD, McElhaney RN. Membrane enzymes: artifacts in Arrhenius plots due to temperature dependence of substrate-binding affinity. Science. 1978;199:902–4. doi: 10.1126/science.146257. [DOI] [PubMed] [Google Scholar]
- [55].Al-Shawi MK, Ketchum CJ, Nakamoto RK. Energy coupling, turnover, and stability of the FoF1 ATP synthase are dependent on the energy of interaction between gamma and beta subunits. J. Biol. Chem. 1997;272:2300–6. doi: 10.1074/jbc.272.4.2300. [DOI] [PubMed] [Google Scholar]
- [56].Baracca A, Amler E, Solaini G, Parenti Castelli G, Lenaz G, Houstek J. Temperature-induced states of isolated F1-ATPase affect catalysis, enzyme conformation and high-affinity nucleotide binding sites. Biochim. Biophys. Acta. 1989;976:77–84. doi: 10.1016/s0005-2728(89)80191-4. [DOI] [PubMed] [Google Scholar]


