Abstract
CD4+ CD25+ T cells are a population of regulatory T cells responsible for the modulation of the immune response in several autoimmune and infectious disease models. We previously showed that adoptive transfer of enriched CD4+ CD25+ T cells also plays a major role in the prevention of arthritis in Borrelia-vaccinated (Borrelia burgdorferi isolate 297) and -challenged (B. bissettii) mice. Here, we present evidence that administration of anti-CD25 antibody at the time of challenge or at later intervals fails to enhance the development of severe destructive osteoarthropathy in Borrelia-vaccinated C57BL mice. However, Borrelia-vaccinated and -challenged mice receiving anti-CD25 antibody developed decreased borreliacidal antibody titers compared to vaccinated and challenged controls. These findings suggest that additional mechanisms besides CD4+ CD25+ T cells are involved in the regulation of the immune response to Borrelia infection following vaccination.
We showed previously that severe destructive arthritis could be induced in Borrelia-vaccinated and -challenged mice (6, 9, 33, 34). The arthritis was prevented when these mice were treated with anti-interleukin 17 (IL-17) antibody (6). Concomitantly, the anti-IL-17-treated Borrelia-vaccinated and -challenged mice developed an inordinate number of CD4+ CD25+ T cells in the lymph nodes adjacent to the arthritic site (33). When anti-IL-17-treated Borrelia-vaccinated and -challenged mice were administered anti-CD25 antibody, the number of CD4+ CD25+ T cells decreased. More importantly, severe destructive arthritis was induced (33). These results suggest that CD4+ CD25+ T cells play an important role in the prevention of arthritis.
The importance of CD4+ CD25+ T cells in prevention of arthritis was further advanced by adoptive transfer studies (34). Highly purified CD4+ CD25+ T cells were obtained from Borrelia-vaccinated and -infected mice treated with anti-IL-17 antibody. When Borrelia-vaccinated and -challenged mice were infused with CD4+ CD25+ T cells, they failed to develop severe destructive arthritis. In contrast, Borrelia-vaccinated and -challenged recipients of CD4+ CD25− T cells developed inflammation of the subsynovial tissues surrounding the tibiotarsal joint, destruction of articular cartilage, synovial hyperplasia, and infiltration of neutrophils into the synovial space. Together, these approaches establish that CD4+ CD25+ T cells can act to control the immunopathology of arthritis.
It is clear that CD4+ CD25+ T cells are essential for maintaining homeostasis of the immune system (1, 48), especially for control of autoimmune diseases (3, 16, 37, 38, 43-45). In addition, the regulatory activity of these naturally occurring CD4+ CD25+ T cells can be modulated by antigen stimulation or exposure to various cytokines (7, 13). Treatment of Borrelia-vaccinated and -challenged mice with anti-IL-17 antibody may have altered the immune system to produce a unique and potent population of CD4+ CD25+ cells that prevent arthritis (33). Here, we present evidence that depletion of CD4+ CD25+ T cells in non-IL-17-treated Borrelia-vaccinated and -challenged mice does not enhance the immunopathology of arthritis, nor does it promote borreliacidal antibody production.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were obtained from William F. Dove (University of Wisconsin), while gamma interferon gene-deficient mice (parental strain C57BL/6) were obtained from W. P. Weidanz (University of Wisconsin) with permission from Genentech, Inc. (South San Francisco, CA). The gamma interferon-deficient mice were used in confirmatory studies. The mice were bred at the animal facility located at the University of Wisconsin Medical School. Six- to 12-week-old inbred male and female mice weighing 20 to 30 g were housed at an ambient temperature of 21°C. Food and acidified water were provided ad libitum during a light and dark cycle of 12 hours. Experimental protocols were reviewed and approved by the Animal Care and Use Committee for the University of Wisconsin Medical School.
Organisms and preparation.
Low-passage (<10) virulent B. burgdorferi 297 (human spinal fluid) and B. bissettii (formerly B. burgdorferi strain C-1-11; from Microtus pennsylvanicus) representing two distinct seroprotective groups among isolates of B. burgdorferi (24) were grown at 32°C in modified Barbour-Stoenner-Kelly (BSK) medium until reaching a concentration of approximately 107 spirochetes/ml. Five-hundred-microliter samples were then dispensed into 1.5-ml screw-cap tubes (Sarstedt, Newton, NC) containing 500 μl of BSK medium supplemented with 10% glycerol (Sigma Chemical Co., St. Louis, MO). The tubes were sealed and stored at −70°C. Six days prior to infection of mice, a frozen suspension of spirochetes was thawed and added to 9 ml of BSK medium and incubated at a temperature of 32°C. On the day of infection, the organisms were visualized by dark-field microscopy and enumerated using a Petroff-Hausser counting chamber.
Vaccine preparation.
Borrelia organisms were grown in 1 liter of BSK medium for 6 days, pelleted by centrifugation (10,000 × g, 15°C, 10 min), and washed three times with phosphate-buffered saline (PBS; pH 7.4). The washed pellet was resuspended in 1% formalin, incubated at 32°C with periodic mixing for 30 min, washed three times by centrifugation with PBS (10,000 × g, 10°C, 15 min), and resuspended in PBS. Subsequently, the formalin-inactivated spirochetes were mixed with a sufficient volume of 1% aluminum hydroxide (Reheis, Berkeley Heights, NJ) to yield 4 × 106 spirochetes/ml.
Vaccination of mice.
Mice were anesthetized with ether contained in a nose-and-mouth cup and injected subcutaneously in the inguinal regions with 0.25 ml of the formalin-inactivated whole-cell vaccine preparation. Whole cells of Borrelia are not recommended for development of a vaccine for humans, based on past concerns associated with other types of whole-cell vaccines (18). However, the ability of whole cells to consistently induce arthritis in mice allows evaluation of immunological mechanisms responsible for the arthritis (6, 9, 33, 34).
Infection of mice.
Twenty-one days after vaccination with B. burgdorferi isolate 297 in alum, mice were anesthetized with ether contained in a nose-and-mouth cup and injected subcutaneously using a 1-ml tuberculin syringe with a 27-gauge needle in both hind footpads with 50 μl of BSK medium containing 106 viable B. bissettii organisms. Some vaccinated mice were also challenged the following day. It was necessary to infect mice with B. bissettii because vaccination with B. burgdorferi isolate 297 induces protective antibodies that prevent the homologous infection from eliciting arthritis (11, 22). Other infectious Borrelia isolates, besides B. bissettii, are also effective in eliciting the arthritis (11, 39). Controls included vaccinated mice injected with BSK medium alone.
Administration of anti-CD25 antibody.
Purified rat anti-mouse CD25 monoclonal antibody (clone PC61; 0.5 mg) was obtained from BD PharMingen (San Diego, CA). The antibody was resuspended in filter-sterilized (0.2-μm-pore-size filter; Acrodisk; Gelman Sciences, Ann Arbor, MI) PBS (pH 7.2) to yield a concentration of 50 μg/ml.
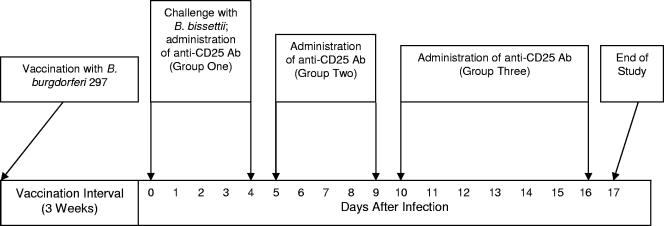
Twenty-one days after vaccination, 16 mice were infected with 106 viable Borrelia organisms in the hind paws. One hour after challenge, one group of four vaccinated and challenged mice was injected in the hind paws with 50 μl (2.5 μg) of anti-CD25 antibody and daily thereafter for 4 days. A second group of four vaccinated and challenged mice received anti-CD25 antibody 5 days after challenge and daily thereafter for 4 days. Finally, a third group of four vaccinated and challenged mice was administered anti-CD25 antibody on day 10 after infection and daily thereafter for 6 days. The remaining four vaccinated and challenged mice were injected with an immunoglobulin G1 (IgG1) isotype control antibody (R&D Systems, Minneapolis, MN).
Flow cytometry.
The inguinal lymph nodes were obtained from untreated and anti-CD25-treated Borrelia-vaccinated and -challenged mice 8 and 20 days after infection. Single-cell suspensions of the lymph node cells were prepared by teasing apart the nodes with forceps and passing them through a sterile nylon mesh screen (Fisher, Hanover Park, IL) into cold filter-sterilized PBS. Suspensions of lymph node cells were placed in chilled centrifuge tubes, and the total number of lymphocytes was determined. Cells (5 × 105) were then dispensed into chilled centrifuge tubes and mixed with 2.5 μl each of fluorescein isothiocyanate-conjugated rat anti-mouse CD4 antibody (BD PharMingen, San Diego, CA) and R-phycoerythrin-conjugated rat anti-mouse CD25 antibody (BD PharMingen) and incubated at 4°C for 30 min under dark conditions. Isotype controls for each antibody (BD PharMingen) were also included.
Subsequently, the cells were washed by centrifugation with PBS at 4°C (500 × g, 5 min) and the pellets were resuspended in 300 μl of cold PBS. Propidium iodide (50 μl of 50 μg/ml; Sigma) was added to each tube to discriminate between viable and nonviable cells. Data were acquired using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) using CellQuest acquisition and analysis (Becton Dickinson). Events were gated to include viable lymphocytes. Ten thousand gated events were collected and analyzed using a quadrant dot plot. Total cell populations for CD4+ and CD4+ CD25+ T cells in the lymph nodes were calculated by multiplying the percentage of occurrence in a dot plot of a cell population by the total number of cells counted in the node.
Preparation of tissues for histological examination.
At 7, 11, and 17 days after infection, mice were euthanized with ether and their hind paws were amputated at mid-femur. The paws were then fixed in 10% neutral-buffered zinc formalin for 24 h. Subsequently, the paws were placed in decalcifying solution (Lerner Laboratories, Pittsburgh, PA) for 24 h, followed by addition of fresh decalcifying solution for an additional 48 h. Following decalcification, the legs were placed in tissue embedding cassettes (Fisher Scientific), embedded in paraffin, and cut into 6-μm-thick sections. The sections were placed on glass slides and stained with hematoxylin and eosin. Sections were cryptically coded, and an unbiased histopathological examination was performed by a board-certified pathologist (T. F. Warner).
Detection of borreliacidal antibodies.
Borreliacidal antibodies were detected by a flow cytometric procedure (8). Viable B. burgdorferi 297 or B. bissettii organisms in logarithmic growth phase were enumerated with a Petroff-Hausser counting chamber and diluted with fresh BSK medium to a concentration of approximately 5 × 105 organisms/ml. Concomitantly, serum samples were diluted 1:20 with BSK and filter sterilized by passage through a 0.2-μm microcentrifuge filter (Costar, Cambridge, MA). The filtered serum samples were then transferred to sterile 1.5-ml screw-cap microcentrifuge tubes (Sarstedt, Newton, NC) and diluted serially (1:40 to 1:40,960) with BSK. Serum samples were heat inactivated at 56°C for 10 min, and a 100-μl aliquot of the spirochetes and 10 μl of sterile guinea pig serum (Sigma; 50% hemolytic component, ≥200 units/ml) were added. The assay mixtures were mixed thoroughly and incubated for 16 to 24 h at 35°C. Following incubation, 100 μl of each assay suspension was transferred to a 12- by 75-mm polystyrene tube (Becton Dickinson, Franklin Lakes, NJ) containing 400 μl of PBS and 1 μg of acridine orange (Sigma) per ml. A FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA) was then used to detect borreliacidal activity. Spirochetes were isolated by gating (CellQuest software; Becton Dickinson) and analyzed for 1 to 2 min with the flow rate set at low. Borreliacidal antibodies kill the spirochete by inducing a complement cascade that disrupts the outer membrane and causes the membrane to bleb. Borreliacidal antibodies were detected indirectly by monitoring the increased fluorescence intensity that occurs when the acridine orange intercalates into blebbed, nonviable spirochetes. A ≥13% shift in the mean fluorescence intensity compared to that of a normal serum control was considered positive (8). The presence of blebbed, nonmotile B. burgdorferi organisms was then confirmed by dark-field microscopy.
Statistical analysis.
Swelling of the hind paws and flow cytometry data among groups were tested by an analysis of variance (42). The Fisher least-significant-difference test was used to examine pairs of means when a significant F ratio indicated reliable mean differences between the control and the various test groups. The alpha level was set at 0.05 before the experiments were started. The standard error for the experiment was then determined.
RESULTS
Effects of anti-CD25 antibody treatment on development of hind paw swelling.
Four groups of four vaccinated mice each were challenged in both hind paws with 106 viable Borrelia organisms 21 and 22 days after vaccination (Fig. 1). Concomitantly, one of the four groups of vaccinated and challenged mice was administered anti-CD25 antibody in both hind paws on the day of initial challenge and daily thereafter for 4 days. Similarly, anti-CD25 antibody was administered to a second vaccinated and challenged group beginning on day 5 after initial challenge and daily thereafter for 4 days. In addition, a third group of vaccinated and challenged mice received anti-CD25 antibody beginning on day 10 after initial infection and daily thereafter for 5 days. The remaining vaccinated and challenged group, not administered anti-CD25 antibody, received an isotype antibody and served as the control group.
FIG. 1.
Schedule of Borrelia vaccination, infection of mice, and treatment with anti-CD25 antibody.
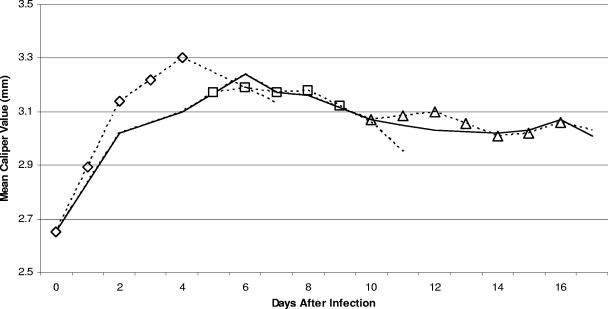
Significant (P ≤ 0.05) swelling of the hind paws was detected with non-anti-CD25 antibody-treated vaccinated and challenged mice, peaking on day 6 after initial challenge and then decreasing (Fig. 2). Administration of anti-CD25 antibody to vaccinated and challenged mice immediately following infection hastened the onset of swelling of the hind paws. However, the swelling declined rapidly and was not significantly different from the swelling of the hind paws of non-anti-CD25 antibody-treated vaccinated and challenged mice. Similarly, no significant differences in hind paw swelling were noted between vaccinated and challenged mice receiving anti-CD25 antibody at the later intervals and swelling of the hind paws of non-anti-CD25 antibody-treated vaccinated and challenged mice.
FIG. 2.
Development of swelling of the hind paws of vaccinated and challenged mice (solid line) and vaccinated and challenged mice administered anti-CD25 antibody immediately following infection (diamonds) or at day 5 (squares) or day 10 (triangles) after initial infection.
Histopathology of anti-CD25 antibody-treated vaccinated and challenged mice.
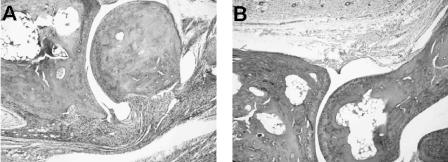
Borrelia-vaccinated and -challenged mice showed edematous changes throughout the hind paws. The synovial space of the tibiotarsal joint contained neutrophils, while the synovium had significant hyperplasia and hypertrophy along with vilus formation. Severe inflammation with many neutrophils along with macrophages and lymphocytes was also detected in the synovium and subsynovium (Fig. 3A). The histopathology of Borrelia-vaccinated and -infected mice treated with anti-CD25 antibody immediately after infection or at days 5 and 10 after infection was not significantly different from the histopathology of Borrelia-vaccinated and -infected mice (Fig. 3B). When these studies were repeated, similar results were observed.
FIG. 3.
Histopathology of the tibiotarsal joints of a vaccinated and challenged mouse (A) and a vaccinated and challenged mouse administered anti-CD25 antibody immediately following challenge (B). Both mice show similar histopathologic changes, including infiltration of neutrophils in the synovium and subsynovial tissues. The remaining mice from both groups had similar histopathological responses. Magnification, ×40.
Anti-CD25 antibody treatment decreased the number of CD4+ CD25+ T cells in local lymph nodes.
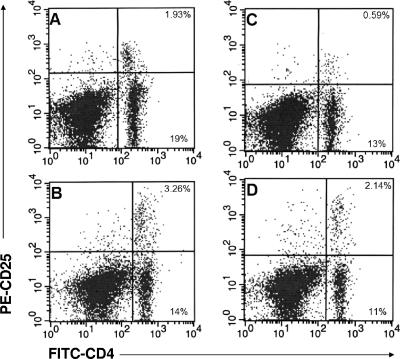
Two groups of six mice each were vaccinated with B. burgdorferi strain 297 and challenged 21 days later with B. bissettii. One of these groups was treated with anti-CD25 antibody on the day of challenge and daily thereafter for 7 days. On days 8 and 20 after challenge, the percentages of CD4+ and CD4+ CD25+ T cells were determined in the inguinal lymph nodes by flow cytometry. Figure 4C shows that anti-CD25 antibody treatment reduced the population of CD4+ CD25+ T cells in inguinal lymph nodes of vaccinated and challenged mice by 69% 8 days after challenge compared to the control (Fig. 4A). By day 20 after challenge (Fig. 4D), the percentage of CD4+ CD25+ T cells in the inguinal lymph nodes of anti-CD25 antibody-treated mice partially recovered but still remained 34% lower than that in untreated vaccinated and challenged mice (Fig. 4B). In addition, anti-CD25 antibody-treated vaccinated and challenged mice had fewer numbers of CD4+ T cells at days 8 or 20 after infection.
FIG. 4.
Numbers of CD4+ CD25+ T cells in the inguinal lymph nodes of Borrelia-vaccinated and -challenged mice without (A and B) and with (C and D) treatment with anti-CD25 antibody at days 8 (A and C) and 20 (B and D) after infection. The upper right quadrants show the percentages of CD4+ CD25+ T cells. The lower right quadrants show the percentages of CD4+ CD25− T cells.
Effects of anti-CD25 antibody on induction of borreliacidal activity against B. bissettii challenge.
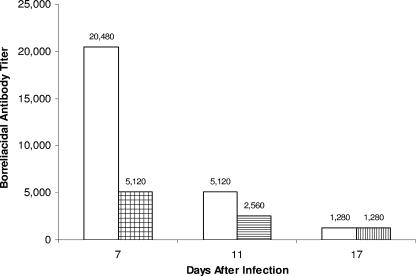
Sera were collected from Borrelia-vaccinated and -challenged mice with or without anti-CD25 antibody treatment. Vaccinated mice were challenged on days 21 and 22 after vaccination. Significant borreliacidal activity against B. bissettii challenge (titer, 20,480) was detected in the sera of Borrelia-vaccinated and -challenged mice 7 days after initial challenge (Fig. 5). By day 11 after challenge, the borreliacidal activity decreased fourfold (titer, 5,120), followed by another fourfold decrease 17 days after challenge. By contrast, Borrelia-vaccinated and -challenged mice receiving anti-CD25 antibody on the day of challenge and daily thereafter for 4 days exhibited significantly reduced borreliacidal activity 7 days after initial challenge (titer, 5,120). Vaccinated and challenged mice administered anti-CD25 antibody daily between days 5 and 9 after challenge also exhibited reduced borreliacidal activity compared to controls 11 days after challenge (titer, 2,560). However, no difference in borreliacidal activity was detected between Borrelia-vaccinated and -challenged mice treated with anti-CD25 antibody between days 10 and 16 after challenge and untreated vaccinated and challenged mice 17 days after initial challenge.
FIG. 5.
Borreliacidal antibody titers of Borrelia-vaccinated and -challenged mice with (shaded boxes) or without (open boxes) treatment with anti-CD25 antibody at days 7, 11, and 17 after infection. Three groups of four mice each of Borrelia-vaccinated and -challenged animals were treated with anti-CD25 antibody from 0 to 4 days, 5 to 9 days, and 10 to 16 days after infection before sera were collected on days 7, 11, and 17 after infection. In most cases (90%), the same titer was obtained when the study was repeated.
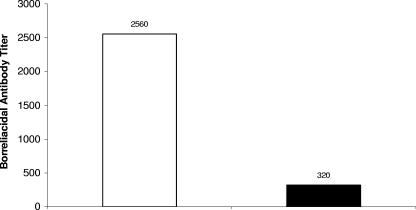
Similar results were obtained when these studies were repeated with vaccinated mice challenged only once with B. bissettii (Fig. 6). Two groups of four mice were vaccinated with B. burgdorferi strain 297 and challenged in the hind paws with B. bissettii 21 days later. One of these two vaccinated and challenged groups was administered anti-CD25 antibody on the day of challenge and daily thereafter for 7 days. Sera were collected from these groups on day 8 after challenge and examined for borreliacidal activity. Figure 6 shows that significant borreliacidal activity against B. bissettii was detected in the sera of untreated vaccinated and challenged mice (titer, 2,560). By contrast, there was an eightfold reduction in the borreliacidal activity (titer, 320) of vaccinated and challenged mice receiving anti-CD25 antibody.
FIG. 6.
Borreliacidal antibody titers of Borrelia-vaccinated and -challenged mice with (shaded box) and without (open box) treatment with anti-CD25 antibody. The same titer was obtained when the study was repeated.
Effects of anti-CD25 antibody on induction of borreliacidal activity against the vaccine isolate, strain 297.
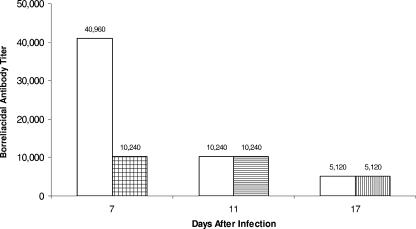
Sera were collected from Borrelia-vaccinated and -challenged mice with or without anti-CD25 antibody treatment. Significant borreliacidal activity against B. burgdorferi strain 297 (titer, 40,960) was detected in sera of Borrelia-vaccinated and -challenged mice 28 days after vaccination (7 days after B. bissettii challenge) (Fig. 7). By day 32 after vaccination (11 days after challenge), the borreliacidal activity decreased significantly (titer, 10,240). By day 38 after vaccination (day 17 after challenge), the titer decreased to 5,120. By contrast, Borrelia-vaccinated and -challenged mice receiving anti-CD25 antibody on the day of challenge and daily thereafter for 4 days exhibited significantly reduced borreliacidal activity to isolate 297 (titer, 10,240) compared to the untreated vaccinated and challenged controls. There was no difference in borreliacidal activity against B. burgdorferi 297 between Borrelia-vaccinated and -challenged mice treated with anti-CD25 antibody at the later intervals and untreated Borrelia-vaccinated and -challenged controls.
FIG. 7.
Effects of anti-CD25 antibody (shaded boxes) on the borreliacidal activity induced by vaccination (open boxes). In most cases (90%), the same titer was obtained when the study was repeated.
DISCUSSION
A subpopulation of CD4+ T cells coexpressing the IL-2 receptor α-chain (CD25) has been shown to play an important role in the prevention of autoimmunity (3, 16, 37, 38, 43-45) and the induction and maintenance of self-tolerance (16, 37, 44, 45). CD4+ CD25+ T regulatory cells also play a major role in down-regulating immune responses (46). Therefore, we texpected the histopathology associated with arthritis in Borrelia-vaccinated and -challenged mice (6, 9, 33, 34) to be exacerbated following treatment with anti-CD25 antibody. We hypothesized that anti-CD25 antibody treatment would deplete (23, 31, 36) or functionally inactivate (19) CD4+ T cells that expressed CD25 constitutively or upon activation (14, 35, 41, 48) by infection of Borrelia-vaccinated mice. The remaining CD4+ CD25− T effector cells would then be free of down-regulation by CD4+ CD25+ T cells to augment the histopathologic responses in anti-CD25 antibody-treated Borrelia-vaccinated and -challenged mice.
Here, we present evidence that depletion of CD4+ CD25+ T cells in Borrelia-vaccinated mice at the time of challenge or later intervals failed to augment the severity of the arthritis compared to nontreated Borrelia-vaccinated and -challenged controls. No significant differences in histopathology of the tibiotarsal joints were detected among the groups, despite administration of anti-CD25 antibody to Borrelia-vaccinated mice at different intervals after Borrelia infection. This finding was unexpected, especially since anti-CD25 antibody-treated Borrelia-vaccinated and -challenged mice had a 69% decrease in the number of CD4+ CD25+ T cells in the lymph nodes near the arthritic site. Moreover, the number of CD4+ CD25+ T cells remained significantly depleted or functionally inactivated (19) throughout the duration of the study, despite early termination of treatment with anti-CD25 antibody. In support of this finding, McHugh and Shevach (27) also showed that depletion of CD25+ T cells was not sufficient for the induction of autoimmunity.
Treatment with anti-CD25 antibody, however, did affect the production of borreliacidal antibody. Borrelia-vaccinated and -challenged mice treated with anti-CD25 antibody developed significantly reduced titers of borreliacidal antibody against the organism of vaccination and challenge. We showed previously that borreliacidal antibody can protect animals from developing arthritis (24). Inhibition of borreliacidal antibody, especially directed against the challenge agent, should prevent elimination of B. bissettii. Immune CD4+ effector T cells would then be continuously stimulated to augment the severity of arthritis in vaccinated mice treated with anti-CD25 antibody. However, no differences were detected in the severity of arthritis between Borrelia-vaccinated and -challenged mice with or without treatment with anti-CD25 antibody. Taken together, these findings suggest that additional mechanisms besides CD4+ CD25+ T cells are involved in the regulation of the immune response to Borrelia infection following vaccination.
Our finding of suppressed borreliacidal antibody differs from those of Eddahri et al. (12). They showed that depletion of CD4+ CD25+ regulatory T cells enhanced the humoral response against a panel of foreign antigens. This discrepancy in the humoral responses after depletion or functional inactivation (19) of CD4+ CD25+ regulatory T cells is important. It points out that we do not know the precise circumstances or immune mechanisms by which antigen-specific T cells control B-cell responses in an environment of CD4+ CD25+ T-cell depletion. Additional studies are needed to define the role that CD4+ CD25+ regulatory T cells have on vaccination and challenge, especially on the induction and maintenance of borreliacidal antibodies, which are required for protection of humans and other animals against infection with B. burgdorferi.
What immune mechanisms could account for these findings? It is known that anti-CD25 antibody binds to the high-affinity α-chain of the IL-2 receptor found on newly activated CD4+ effector cells, CD25+ constitutively expressing CD4+ T cells, and regulatory cells (4, 17, 19, 25, 28, 30). Binding of anti-CD25 antibody to the IL-2α receptors of both effector and regulatory cells would limit their proliferation (34). Presumably, administration of anti-CD25 antibody to Borrelia-vaccinated and -challenged mice would prevent newly activated CD25 effector T cells or constitutively expressing CD25 effector cells from receiving IL-2. The lack of binding of IL-2 to the CD25 α-receptor would prevent these cells from proliferating and exerting their arthritic activity. This would account for the failure of anti-CD25 treatment to exacerbate the severity of the arthritis in Borrelia-vaccinated and -challenged mice, even in an environment with reduced regulatory T-cell activity.
Although anti-CD25 antibody can block IL-2 binding to the IL-2α receptor, anti-CD25 antibody-treated Borrelia-vaccinated and -challenged mice still develop arthritis. We speculate that blockage of the IL-2α receptor does not prevent IL-2 from binding to the IL-2β or -γ receptor chains of the IL-2 receptor complex (34, 47). In addition, IL-15 is a known T-cell proliferating cytokine that can bind to the IL-2β receptor. We showed previously that treatment of Borrelia-vaccinated and -challenged mice with anti-IL-15 antibody prevented arthritis (2). In the absence of IL-2 binding to the IL-2α receptor, both IL-2 and IL-15 could bind to the IL-2β chain and induce proliferation or activation of T effector cells to drive the arthritis. Additional studies are needed to define whether these secondary receptors are involved in the induction of arthritis associated with Borrelia vaccination and challenge.
Another immune mechanism involving CD25 may affect borreliacidal antibody production. Borrelia-vaccinated mice are actively making an antibody response to different Borrelia components in the vaccine at the time of infection (40) and initial treatment with anti-CD25 antibody. In this report, we showed that treatment with anti-CD25 antibody caused a fourfold or more decrease in borreliacidal antibody against both the agent of vaccination (isolate 297) and challenge (B. bissettii). It is known that B cells and macrophages express CD25 (10, 15, 21, 31, 32). Depletion or inactivation of CD25-expressing B cells would result in a reduction of the borreliacidal titer to both the vaccine and challenge organisms. Likewise, treatment with anti-CD25 antibody may reduce the effectiveness of macrophages processing the challenge agent. It is important to note that treatment with anti-CD25 antibody at later intervals after infection had only a minor or no effect on borreliacidal activity. It is possible that CD25 expression on B cells or macrophages is a marker for early antibody production. Once CD25 expression or receptivity to IL-2 is down-regulated, antibody production decreases and treatment with anti-CD25 antibody has no effect on the declining borreliacidal antibody production. Finally, injection of anti-CD25 antibody may reduce CD4+ effector T cells that constitutively express CD25. We did show that anti-CD25 treatment not only reduced the number of cells expressing CD4+ CD25+ but also reduced the total number of CD4+ T cells. The reduction of CD4+ T cells may have been due to depletion of CD4+ T cells that express low concentrations of CD25. Depletion of these effector cells would affect antibody production. In support of this latter theory, CD4+ T cells expressing low concentrations of CD25 have been linked to activation of the immune response, while CD4+ T cells expressing high or stable concentrations of CD25 have regulatory activity (5, 20, 29).
The failure of anti-CD25 antibody treatment to exacerbate arthritis in Borrelia-vaccinated and -challenged mice conflicts with our previous findings (33). Recently, we showed that Borrelia-vaccinated and -challenged mice treated with anti-IL-17 antibody failed to develop severe, destructive arthritis (33). Moreover, these mice developed an inordinate number of CD4+ CD25+ T cells in the lymph nodes. The CD4+ CD25+ T cells represented 25% of the lymph node population. Normally, only 5 to 10% of circulating lymphocytes are CD4+ CD25+ T cells (26). When these anti-IL-17-treated vaccinated and challenged mice were administered anti-CD25 antibody, they developed a severe osteoarthropathy, including significant erosion of bone and cartilage (33). Moreover, the number of CD4+ CD25+ T cells in the lymph nodes decreased 10-fold or more following treatment with anti-CD25 antibody. It seems reasonable that the drastic reduction in CD4+ CD25+ T cells limited their suppressive activity. By contrast, anti-CD25 antibody treatment of Borrelia-vaccinated and -challenged mice had no effect on the arthritis and did not remarkably change the ratio between effector and regulatory cells. It is likely that immune suppression enforced by CD4+ CD25+ T cells is not an all-or-nothing response but rather a unique balance between the number of CD4+ CD25− T cells and the number of CD4+ CD25+ T regulatory cells.
In summary, we have shown that administration of anti-CD25 antibody to Borrelia-vaccinated and -challenged mice at the time of infection does not exacerbate the osteoarthropathy observed in vaccinated and challenged controls. However, borreliacidal antibody production was decreased in the anti-CD25 antibody-treated Borrelia-vaccinated and -challenged mice. These findings suggest that additional mechanisms besides CD4+ CD25+ T cells are involved in the regulation of the immune response to Borrelia infection following vaccination. The availability of a reproducible mouse model of Lyme-associated arthritis permits development of additional approaches by which to define the immune mechanisms responsible for the prevention and development of arthritis.
Acknowledgments
This study was supported by the Wisconsin State Laboratory of Hygiene, the public health laboratory for the state of Wisconsin, Madison, WI, and the Gundersen Medical Foundation, La Crosse, WI.
We also thank the Flow Cytometry Facility at the University of Wisconsin Hospital (Madison, WI) as well as Dean Jobe and Krista Asp for their assistance in performing the borreliacidal assays.
REFERENCES
- 1.Almeida, A. R., N Legrand, M. Papiernik, and A. A. Freitas. 2002. Homeostasis of peripheral CD4+ T cells: IL-2Rα and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J. Immunol. 164:4850-4860. [DOI] [PubMed] [Google Scholar]
- 2.Amlong, C. A., D. T. Nardelli, S. H. Peterson, T. F. Warner, S. M. Callister, and R. F. Schell. 2006. Anti-interleukin-15 prevents arthritis in Borrelia-vaccinated and infected mice. Clin. Vaccine Immunol. 13:289-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asano, M., M. Toda, N. Sakaguchi, and S. Sakaguchi. 1996. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 184:387-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baan, C. C., I. C. van Riemsdijk-Overbeeke, M. J. A. M. Boelaars-van Haperen, J. M. N. Ijzermans, and W. Weimar. 2002. Inhibition of the IL-15 pathway in anti-CD25 mAb treated renal allograft recipients. Transplant Immunol. 10:81-87. [DOI] [PubMed] [Google Scholar]
- 5.Baecher-Allan, C., J. A. Brown, G. J. Freeman, and D. A. Hafler. 2001. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167:1245-1253. [DOI] [PubMed] [Google Scholar]
- 6.Burchill, M. A., D. T. Nardelli, D. M. England, D. J. DeCoster, J. A. Christopherson, S. M. Callister, and R. F. Schell. 2003. Inhibition of interleukin-17 prevents the development of arthritis in vaccinated mice challenged with Borrelia burgdorferi. Infect. Immun. 71:3437-3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bushell, A., E. Jones, A. Gallimore, and K. Wood. 2005. The generation of CD4+CD25+ regulatory T cells that prevent allograft rejection does not compromise immunity to a viral pathogen. J. Immunol. 174:3290-3297. [DOI] [PubMed] [Google Scholar]
- 8.Callister, S. M., D. A. Jobe, R. F. Schell, C. S. Pavia, and S. D. Lovrich. 1996. Sensitivity and specificity of the borreliacidal antibody test during early Lyme disease: a “gold standard”? Clin. Diagn. Lab. Immunol. 3:399-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christopherson, J. A., E. L. Munson, D. M. England, C. L. Croke, M. C. Remington, M. L. Molitor, D. J. DeCoster, S. M. Callister, and R. F. Schell. 2003. Destructive arthritis in vaccinated interferon gamma-deficient mice challenged with Borrelia burgdorferi: modulation by tumor necrosis factor alpha. Clin. Diagn. Lab. Immunol. 10:44-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox, G. W., B. J. Mathieson, S. L. Giardina, and L. Varesio. 1990. Characterization of IL-2 receptor expression and function on murine macrophages. J. Immunol. 145:1719-1726. [PubMed] [Google Scholar]
- 11.Croke, C. L., E. L. Munson, S. D. Lovrich, J. A. Christopherson, M. C. Remington, D. M. England, S. M. Callister, and R. F. Schell. 2000. Occurrence of severe destructive Lyme arthritis in hamsters vaccinated with outer surface protein A and challenged with Borrelia burgdorferi. Infect. Immun. 68:658-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eddahri, F., G. Oldenhove, S. Denanglaire, J. Urbain, O. Leo, and F. Andris. 2006. CD4+CD25+ regulatory T cells control the magnitude of T-dependent humoral immune responses to exogenous antigens. Eur. J. Immunol. 36:855-863. [DOI] [PubMed] [Google Scholar]
- 13.Fehervari, Z., and S. Sakaguchi. 2005. CD4+ regulatory cells as a potential immunotherapy. Philos. Trans. R. Soc. Lond. B 360:1647-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goleva, E., I. D. Cardona, L. Ou, and D. Y. M. Leung. 2005. Factors that regulate naturally occurring T regulatory cell-mediated suppression. J. Allergy Clin. Immunol. 116:1094-1100. [DOI] [PubMed] [Google Scholar]
- 15.Hancock, W. W., W. A. Muller, and R. S. Cotran. 1987. Interleukin 2 receptors are expressed by alveolar macrophages during pulmonary sarcoidosis and are inducible by lymphokine treatment of normal human lung macrophages, blood monocytes, and monocyte cell lines. J. Immunol. 138:185-191. [PubMed] [Google Scholar]
- 16.Itoh, M., T. Takahashi, N. Sakaguchi, Y. Kuniyasu, J. Shimizu, F. Otsuka, and S. Sakaguchi. 1999. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 162:5317-5326. [PubMed] [Google Scholar]
- 17.Jones, E., M. Dahm-Vicker, A. K. Simon, A. Green, F. Powrie, V. Cerundolo, and A. Gallimore. 2002. Depletion of CD25+ regulatory cells results in suppression of melanoma growth and induction of autoreactivity in mice. Cancer Immunol. 2:1-10. [PubMed] [Google Scholar]
- 18.Keitel, W. A. 1999. Cellular and acellular pertussis vaccines in adults. Clin. Infect. Dis. 28(Suppl. 2):S118-S123. [DOI] [PubMed] [Google Scholar]
- 19.Kohm, A. P., J. S. McMahon, J. R. Podojil, W. Smith Begolka, M. DeGutes, D. J. Kasprowicz, S. F. Ziegler, and S. D. Miller. 2006. Cutting edge: anti-CD25 monoclonal antibody injection results in functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J. Immunol. 176:3301-3305. [DOI] [PubMed] [Google Scholar]
- 20.Kuniyasu, Y., T. Takahashi, M. Itoh, J. Schmizu, G. Toda, and S. Sakaguchi. 2000. Naturally anergic and suppressive CD25+CD4+ T cells as a functionally and phenotypically distinct immunoregulatory T cell subpopulation. Int. Immunol. 12:1145-1155. [DOI] [PubMed] [Google Scholar]
- 21.Lewis, D. E., and G. R. Harriman. 2001. Cells and tissues of the immune system, p. 2.1-2.21. In R. R. Rich, T. A. Fleiser, W. T. Shearer, B. L. Kotzin, and H. W. Schroeder, Jr. (ed.), Clinical immunology principles and practice. Mosby, London, England.
- 22.Lim, L. C. L., D. M. England, B. K. DuChateau, N. J. Glowacki, J. R. Creson, S. D. Lovrich, S. M. Callister, D. A. Jobe, and R. F. Schell. 1994. Development of destructive arthritis in vaccinated hamsters challenged with Borrelia burgdorferi. Infect. Immun. 62:2825-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loughry, A., S. Fairchild, N. Athanasou, J. Edwards, and F. C. Hall. 2005. Inflammatory arthritis and dermatitis in thymectomized, CD25+ cell-depleted mice. Rheumatology 44:299-308. [DOI] [PubMed] [Google Scholar]
- 24.Lovrich, S. D., S. M. Callister, L. C. L. Lim, and R. F. Schell. 1993. Seroprotective groups among isolates of Borrelia burgdorferi. Infect. Immun. 61:4367-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malek, T. R., and A. L. Bayer. 2004. Tolerance, not immunity, crucially depends on IL-2. Nat. Rev. Immunol. 4:665-674. [DOI] [PubMed] [Google Scholar]
- 26.Maloy, K. J., and F. Powrie. 2001. Regulatory T cells in the control of immune pathology. Nat. Immunol. 2:816-822. [DOI] [PubMed] [Google Scholar]
- 27.McHugh, R. S., and E. M. Shevach. 2002. Cutting edge: depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J. Immunol. 168:5979-5983. [DOI] [PubMed] [Google Scholar]
- 28.Minami, Y., T. Kono, T. Miyazaki, and T. Taniguchi. 1993. The IL-2 receptor complex: its structure, function, and target gene. Annu. Rev. Immunol. 11:245-267. [DOI] [PubMed] [Google Scholar]
- 29.Miyara, M., Z. Amoura, C. Parizot, C. Badoual, K. Dorgham, S. Trad, M. Kambouchner, D. Valeyre, C. Chapelon-Abric, P. Debré, J.-C. Piette, and G. Gorochov. 2006. The immune paradox of sarcoidosis and regulatory T cells. J. Exp. Med. 203:359-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore, A. C., A. Gallimore, S. J. Draper, K. R. Watkins, S. C. Gilbert, and A. V. S. Hill. 2005. Anti-CD25 antibody enhancement of vaccine-induced immunogenicity: increased durable cellular immunity with reduced immunodominance. J. Immunol. 175:7264-7273. [DOI] [PubMed] [Google Scholar]
- 31.Morgan, M. E., R. P. M. Sutmuller, H. J. Witteveen, L. M. van Duivenvoorde, E. Zanelli, C. J. M. Melief, A. Snijders, R. Offringa, R. R. P. de Vries, and R. E. M. Toes. 2003. CD25+ T cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 48:1452-1460. [DOI] [PubMed] [Google Scholar]
- 32.Nakanishi, K., S. Hirose, T. Yoshimoto, H. Ishizashi, K. Hiroishi, T. Tanaka, T. Kono, M. Miyasaka, T. Taniguchi, and K. Higashino. 1991. Role and regulation of the interleukin (IL)-2 receptor α and β chains in IL-2-driven B-cell growth. Proc. Natl. Acad. Sci. USA 89:3551-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nardelli, D. T., J. P. Cloute, K. H. K. Luk, J. Torrealba, T. F. Warner, S. M. Callister, and R. F. Schell. 2005. CD4+ CD25+ T cells prevent arthritis associated with Borrelia vaccination and infection. Clin. Diagn. Lab. Immunol. 12:786-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nardelli, D. T., M. A. Burchill, D. M. England, J. Torrealba, S. M. Callister, and R. F. Schell. 2004. Association of CD4+ CD25+ T cells with prevention of severe destructive arthritis in Borrelia burgdorferi-vaccinated and challenged interferon gamma-deficient mice treated with anti-interleukin-17 antibody. Clin. Diagn. Lab. Immunol. 11:1075-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson, B. H. 2004. IL-2, regulatory T cells, and tolerance. J. Immunol. 172:3983-3988. [DOI] [PubMed] [Google Scholar]
- 36.Onizuka, S., I. Tawara, J. Shimizu, S. Sakaguchi, T. Fujita, and E. Nakayama. 1999. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor α) monoclonal antibody. Cancer Res. 59:3128-3133. [PubMed] [Google Scholar]
- 37.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance by activated T cells expressing IL-2 receptor α-chain (CD25). J. Immunol. 155:1151-1164. [PubMed] [Google Scholar]
- 38.Salomon, B., D. J. Lenschow, L. Rhee, N. Ashourian, B. Singh, A. Sharpe, and J. A. Bluestone. 2000. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 12:431-440. [DOI] [PubMed] [Google Scholar]
- 39.Schmitz, J. L., R. F. Schell, A. Hejka, D. M. England, and L. Konick. 1998. Induction of Lyme arthritis in LSH hamsters. Infect. Immun. 56:2336-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmitz, J. L., R. F. Schell, S. D. Lovrich, S. M. Callister, and J. E. Coe. 1991. Characterization of the protective antibody response to Borrelia burgdorferi in experimentally infected LSH hamsters. Infect. Immun. 59:1916-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shevach, E. M. 2004. Regulatory/suppressor T cells in health and disease. Arthritis Rheum. 50:2721-2724. [DOI] [PubMed] [Google Scholar]
- 42.Steel, R. G. D., and J. H. Torrie. 1960. Principles and procedures of statistics with special reference to the biological sciences, p. 99-276. McGraw-Hill Book Co., New York, N.Y.
- 43.Suri-Payer, E., A. Z. Amar, A. M. Thornton, and E. M. Shevach. 1998. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 160:1212-1218. [PubMed] [Google Scholar]
- 44.Takahashi, T., T. Tagami, S. Yamazaki, T. Uede, J. Shimizu, N. Sakaguchi, T. W. Mak, and S. Sakaguchi. 2000. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 192:303-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahashi, T., Y. Kuniyasu, M. Toda, N. Sakaguchi, M. Itoh, M. Iwata, J. Shimizu, and S. Sakaguchi. 1998. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10:1969-1980. [DOI] [PubMed] [Google Scholar]
- 46.Trenado, A., M. Sudres, Q. Tang, S. Maury, F. Charlotte, S. Gregoire, M. Bonyhadi, D. Klatzman, B. L. Salomon, and J. L. Cohen. 2006. Ex vivo-expanded CD4+CD25+ immunoregulatory T cells prevent graft-versus-host disease by inhibiting activation/differentiation of pathogenic T cells. J. Immunol. 176:1266-1273. [DOI] [PubMed] [Google Scholar]
- 47.Wang, X., M. Rickert, and K. C. Garcia. 2005. Structure of the quaternary complex of interleukin-2 with its α, β and γC receptors. Science 310:1159-1163. [DOI] [PubMed] [Google Scholar]
- 48.Zelenay, S., T. Lopes-Carvalho, I. Caramalho, M. F. Moraes-Fontes, M. Rebelo, and J. Demengeot. 2005. Foxp3+ CD25− CD4 T cells constitute a reservoir of committed regulatory cells that regain CD25 expression upon homeostatic expansion. Proc. Natl. Acad. Sci. USA 102:4091-4096. [DOI] [PMC free article] [PubMed] [Google Scholar]