Abstract
Human recombinant Fab fragments specific for the spike protein of severe acute respiratory syndrome coronavirus (SARS-CoV) were screened from a human Fab library, which was generated from RNAs from peripheral lymphocytes of convalescent SARS patients. Among 50 randomly picked clones, 12 Fabs specially reacted with S protein by an enzyme-linked immunosorbent assay. The microneutralizing test showed that one clone, designated M1A, had neutralizing activity on Vero E6 cells against SARS-CoV. DNA sequence analysis indicated that the light- and heavy-chain genes of M1A Fab belong to the κ2a and 4f families, respectively. A neutralizing test on purified M1A demonstrated that 0.5 mg/ml of M1A completely inhibited SARS-CoV activity, with an absence of cytopathic effect for 7 days. Real-time fluorescence reverse transcription-PCR also proved the neutralizing capacity of M1A. These data showed that the number of virus copies was significantly reduced in the M1A-treated group, suggesting an important role for M1A in passive immunoprophylaxis against the SARS virus.
During 2002 to 2003, severe acute respiratory syndrome (SARS) broke out worldwide. There is no effective medicine to cure this disease. Previous studies showed SARS patients could make great progress if they were given serum from convalescent SARS patients, indicating that generation of a human monoclonal antibody (MAb) against SARS coronavirus (SARS-CoV) may be helpful for passive immunoprophylaxis against SARS virus (8). The spike protein (S protein) of SARS-CoV is an important target for neutralizing antibody (9). We constructed a human antibody library by the phage display technique and used the S protein of SARS-CoV as the target to screen the phage antibody library. This study describes the generation of one neutralizing SARS-CoV-specific human antibody Fab molecule by panning selection of combinatorial Fab libraries against S proteins and quantitative analysis of the binding and neutralizing activity of the Fab molecules.
MATERIALS AND METHODS
Construction of a Fab phage display library.
Total RNA was extracted from peripheral blood lymphocytes of convalescent SARS patients (RNA isolation kit; Stratagene), and mRNA was reverse transcribed into cDNA using an oligo(dT) primer (Gibco/BRL). Fd segment (variable and first constant domains) genes and light-chain genes were amplified by using primers specific for the human chain genes (1). Then the amplified chains were cloned into the pComb3 phage display vector as described by Bjorling et al. (4). The ligated products were transformed into Escherichia coli XL1-Blue and resulted in a library of 2 × 106 clones. The transformants were expanded into a volume of 2 liters, and the resulting phage was recovered as described previously (4).
Enrichment of antigen-binding clones by panning.
Microtiter wells were coated overnight at 4°C with 50 μl of purified SARS-CoV (Beijing 01 strain) lysate antigen (30 μg/ml), and after a blocking step with 3% bovine serum albumin (BSA)-phosphate-buffered saline (PBS), 50 μl Fab phages (1014 CFU/ml) was added to each well, followed by incubation at 37°C for 2 h. The unbound clones were washed out with a phosphate-buffered saline solution containing 0.02% Tween 20; the wells were washed once at the first round of panning, 5 times at the second round, and 10 and 20 times at the third and fourth rounds, respectively. Bound phages were eluted with 0.1 M triethylamine (pH 2.3) and then immediately neutralized with 2 M Tris base. The eluted phages were amplified by infection of Escherichia coli XL1-Blue cultures, followed by superinfection with helper phage VCS M13 (1012 PFU). For the third and fourth rounds of biopanning, the phage library was biopanned by recombinant spike protein of SARS-CoV, which was expressed in 293T cells and stored at −70°C until use (11). After the last round of panning, phagemid DNA was extracted from the overnight culture, and the vector was modified by excision of the coat protein III-encoding gene fragment of the phage. Propagation and induction of Fab production were performed essentially as previously described (2, 3, 13, 17), and the bacterial supernatants were selected by an enzyme-linked immunosorbent assay (ELISA) against S protein for the presence of S-specific Fabs.
ELISA.
Screening of specific Fab clones against S protein was carried out by a double antibody sandwich ELISA. The protocol was as follows. A rabbit anti-S serum was diluted in 50 mM sodium carbonate buffer (pH 9.6) and applied to microtiter plates, which were left overnight at 4°C. At the same time, plates were coated with BSA and Vero E6 cell lysates as negative antigen controls. Coated plates were blocked with 3% BSA in PBS. A SARS-CoV lysate antigen was added to capture S protein. The plates were then incubated with 50 μl of a crude Fab preparation in PBS at 37°C for 2 h. For the titration study of the binding activity of purified M1A (the neutralizing Fab clone) to S protein, the plates were coated with recombinant S protein expressed in 293T cells, and after blocking with 3% BSA-PBS, twofold serial dilutions of purified Fab ranging from 0.025 mg/ml to 0.2 mg/ml were added. The binding activity of Fabs was detected by a horseradish peroxidase-labeled goat anti-human Fab antibody. Absorbance was measured as the optical density at 450 nm (5, 7).
Virus neutralization capacity assay.
The ability of Fabs to inhibit SARS virus infectivity was assayed by a microneutralization test (MNT). Crude Fabs or twofold dilutions (1 mg/ml to 0.0625 mg/ml) of the purified neutralizing clone M1A were mixed with an equal volume of SARS-CoV at 2,000 PFU/ml. The mixture was incubated for 1 h at 37°C and subsequently inoculated (100 μl) into 96-well tissue culture plates containing confluent Vero E6 cell monolayers. After adsorption for 1 h at 37°C, wells were first overlaid with 100 μl Dulbecco's modified Eagle medium supplemented with 2% fetal calf serum and 20 mM HEPES and then incubated at 37°C under a humidified 5% CO2 atmosphere. After 2 days of incubation, an inverted microscope was used to examine cells for the appearance of cytopathic effect (CPE) for 5 more days. The neutralization effect was determined by inhibition of CPE in wells containing Fab molecules, and the titer end point of M1A was taken as the highest dilution that completely neutralized the infective dose of virus. As a negative control, the supernatant of pComb3 vector culture was included, and the neutralization assay was repeated at least twice, with reproducible results (19).
Sequence analysis.
The nucleotide sequences of the VL and VH regions were determined by the T7 primer (5′-GTAATACGACTCACTATAGGG-3′) and the T3 primer (5′-AATTAACCCTCACTAAAG-3′) on the pComb3 sequence, respectively (16).
Expression and purification of M1A protein.
The VH and VL gene fragments of M1A were cloned into the PET32a vector and expressed in E. coli BL21(DE3). Protein products of the Fab were purified from both the pellet and the supernatant of the culture by affinity chromatography. The affinity column was generated by linking goat anti-human immunoglobulin G (IgG) to CNBr-activated Sepharose 4 according to the manufacturer's instructions (Pharmacia Biotech). Then crude Fab preparations were diluted in PBS and applied to the column. Bound Fab molecules were eluted with 0.1 M HCl-glycine (pH 2.2) and neutralized immediately with 2 M Tris base. Appropriate Fab-containing fractions were pooled and dialyzed overnight. The resulting Fab preparations were analyzed for Fab concentration, antigen reactivity, and neutralizing activity against SARS-CoV (6).
Testing of neutralizing activity by real-time RT-PCR.
To draw the standard curve and examine the feasibility of a real-time reverse transcription-PCR (RT-PCR) assay for testing the neutralizing capacity of M1A, known amounts of SARS-CoV RNA (0.1, 1, 10, 100, 1,000, and 10,000 PFU) were extracted and subjected to real-time RT-PCR first. Then total RNAs of a virus control group and a test group with M1A (0.5 mg/ml) were isolated from aliquots of culture supernatants, from the first day to the sixth day after virus infection. The real-time RT-PCR assay was performed by following the operation instructions for the novel coronavirus PCR fluorescence diagnostic kit (Piji Biological Co.), and amplification conditions were 42°C for 30 min and 95°C for 3 min, followed by 5 cycles of 95°C for 10 s, 55°C for 30 s, and 72°C for 60 s, and then 40 cycles of 95°C for 5 s and 60°C for 30 s. An ABI 7900 sequence detector was used to analyze the fluorescence emitted during amplification. The relationship between the cycle threshold (CT) and the known number of virus copies used per reaction was drawn by a standard curve. From the standard curve, the virus copies of control groups and test groups can be calculated by the software (21).
RESULTS
Isolation of Fab forms of antibody with neutralizing activity toward SARS-CoV.
After four rounds of panning, 50 colonies were randomly picked up for collection of crude Fabs. The supernatant that contained crude Fab was directly subjected to analysis of binding activity by means of a double antibody sandwich ELISA. The Fab clones specific for S protein were selected by an optical density at 450 nm of >0.5 and a positive/negative ratio of ≥2. Among the 50 clones analyzed, 12 clones appeared to exhibit binding activities specific for S protein. Then the neutralizing capacities of these 12 Fab clones were tested by MNT and were determined by evaluating the percentage of cells showing CPE compared with that in the virus control wells. When the cells were observed by inverted microscope on day 2 of incubation, a specific CPE consisting of rounding and sloughing of infected cells off the monolayer was seen in virus control wells, occurring on about 75% of the cells. By day 3, the changes were very clear-cut; the cell sheet had become almost completely disrupted and consisted of an open meshwork of distorted cells, and CPE occurred on 100% of cells. But in the test group, in the wells containing Fab MIA and virus, the percentage of CPE was much lower than that in the virus control group: 20% of cells showed CPE on day 2, and 75% showed CPE on day 3 (Table 1). This finding indicated that crude M1A could partially neutralize the activity of SARS-CoV by reducing CPE 50% after 2 days of infection with 100 PFU SARS-CoV.
TABLE 1.
Neutralizing test of M1A
| Time | % of cellsa showing CPE
|
|||
|---|---|---|---|---|
| Normal cells | Virus control group | Test group | Negative-control group | |
| Day 2 | 75 | 20 | 75 | |
| Day 3 | 100 | 75 | 100 | |
Normal cells, cells only; virus control group, cells treated with 100 PFU SARS-CoV; test group, cells treated with 100 PFU SARS-CoV and Fab molecule mixture; negative-control group, cells treated with 100 PFU SARS-CoV and pComb3 vector culture.
Sequencing analysis.
DNA sequence analysis confirmed that the light- and heavy-chain genes of M1A Fab belong to the κ2a and 4f families, respectively. The amino acid sequence of the variable region deduced from the DNA sequence is shown in Table 2.
TABLE 2.
Amino acid sequences of the variable regions of M1A Fab
| Chain | Sequence of:
|
||
|---|---|---|---|
| CDR1 | CDR2 | CDR3 | |
| VH | NYGMH | VIWSDGSNKYYGDSVKG | DPVVVVINGDEAFDI |
| VL | RTSQNIGTWLA | DASKRAA | QQRLQWATEN |
Expression and purification of M1A protein.
Since the use of crude Fab in the MNT assay gave weak neutralizing activity due to the low content of Fab in the E. coli culture, M1A protein was expressed in 200 ml of culture and purified by affinity chromatography in order to confirm and quantitatively analyze the neutralizing capacity. A nonreducing polyacrylamide gel electrophoresis assay (Fig. 1A) showed that M1A was not degraded and ran as a single band with the expected Mr of 48,000. Use of an anti-human Fab antibody confirmed that the majority of Fab was present in the eluted fractions (Fig. 1B). After concentration, the final yield of purified M1A was 10 mg (equivalent to 50 mg/liter bacterial supernatant).
FIG. 1.
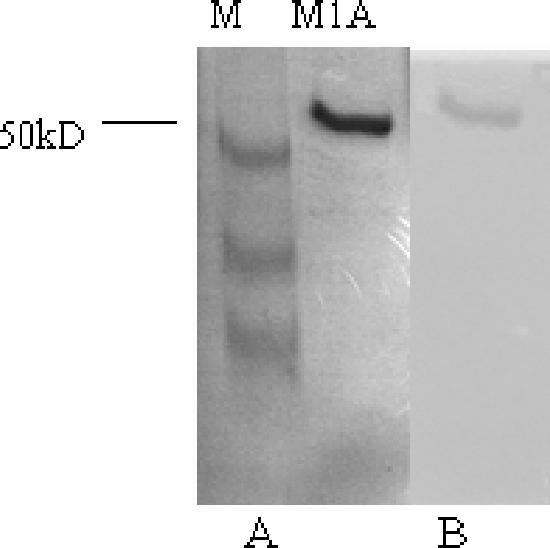
(A) Nonreducing polyacrylamide gel electrophoresis of fractions eluted from an affinity chromatography column. (B) Western blot of a duplicate gel stained with an anti-human Fab antibody.
Analysis of antigen binding activity of purified M1A protein by ELISA.
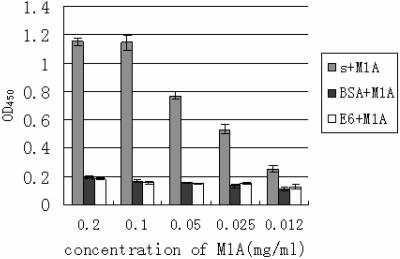
To identify the binding specificity of the Fab molecule M1A to S protein, an ELISA was performed by coating the wells with recombinant S protein as a test antigen. E6 cell lysates and BSA were used as the negative antigen controls. Figure 2 shows that M1A had much stronger reactivity to S protein than to the control antigens.
FIG. 2.
Reactivity of M1A to S protein determined by ELISA. s + M1A, plates were coated with recombinant S protein as an antigen to test the reactivity of M1A. BSA + M1A or E6 + M1A, plates were coated with BSA (1 mg/ml) or Vero E6 cell lysates, respectively, as a negative antigen control to test the cross-reactivity of M1A. OD450, optical density at 450 nm.
Analysis of neutralizing capacity of purified M1A toward SARS-CoV.
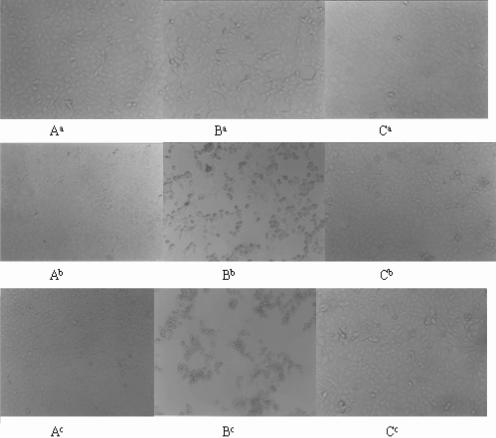
Before the microneutralization test, an MTT assay was utilized to test the effect of M1A on Vero E6 cell proliferation. Cells treated with M1A diluted at 1 mg/ml to 0.0625 mg/ml showed no difference from the control (data not shown). Then serial dilutions (1 mg/ml to 0.0625 mg/ml) of M1A were subjected to the MNT. The neutralization capacity of purified M1A was determined by observing and comparing the difference between CPE appearing on the test group and the virus control group for 6 more days. The appearance of CPE on cells is shown in Fig. 3 and summarized in Table 3. Figure 3 and Table 3 demonstrate that CPE appeared on 50% of cells in the virus control group on day 2 and 100% of cells on day 3 after infection with 100 PFU of SARS-CoV. By exposure to 0.25 mg/ml M1A, cells showed no CPE on days 2 and 3 after infection but 20% CPE on day 4 and 100% CPE on day 5. When cells were exposed to 0.5 mg/ml M1A, no cells showed CPE for 6 more days after infection with 100 PFU of virus. These findings indicate that M1A displayed neutralization capacity starting from a concentration of 0.25 mg/ml, which postponed the occurrence of CPE for 2 days. The titer end point of M1A is 0.5 mg/ml, which completely neutralized the activity of SARS-CoV, with no CPE appearing for 6 more days.
FIG. 3.
Analysis of the neutralizing capacity of purified M1A by MNT. (A) Vero E6 cell control; (B) cells treated with 100 PFU of SARS-CoV; (C) cells treated with 100 PFU of SARS-CoV and 0.5 mg/ml M1A Fab. a, b, and c, CPE of cells on the 2nd, 3rd, and 6th days of virus infection, respectively.
TABLE 3.
Analysis of neutralizing capacity of purified M1A toward SARS-CoV
| Time | % of cellsa with CPE
|
|||||
|---|---|---|---|---|---|---|
| Normal cells | Test group with the following concn of M1A (mg/ml)
|
Virus control group | ||||
| 1 | 0.5 | 0.25 | 0.03125-0.125 | |||
| Day 1 | ||||||
| Day 2 | 50 | 50 | ||||
| Day 3 | 100 | 100 | ||||
| Day 4 | 20 | 100 | 100 | |||
| Day 5 | 100 | 100 | 100 | |||
| Day 6 | 100 | 100 | 100 | |||
Normal cells, cells only; virus control group, cells treated with 100 PFU SARS-CoV; test group, cells treated with 100 PFU SARS-CoV and M1A Fab molecule mixture.
Neutralizing capacity detected by a real-time RT-PCR assay.
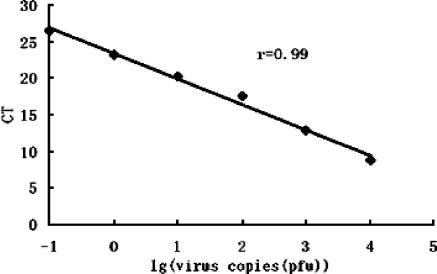
The feasibility of a real-time RT-PCR assay to test the neutralizing capacity of M1A was examined before detection of the virus copies of the control group and the test group with 0.5 mg/ml M1A. A linear standard curve was obtained by subjecting known amounts of SARS-CoV RNA to real-time RT-PCR. The result indicated that as the amount of input RNA increased, the CT required to reach the threshold for fluorescence detection decreased, so a linear relationship was found between the CT and the RNA copy number determined (correlation coefficient [r], 0.99), as shown in Fig. 4, indicating the accuracy of this assay. The assay strongly supported the notion that the results of real-time-RT-PCR could sensitively reflect the number of virus copies used for each reaction.
FIG. 4.
Relationship of known input virus copies to the CT in the real-time RT-PCR assay. r, correlation coefficient.
The RNAs of the virus control group and the test group with 0.5 mg/ml M1A were collected and subjected to a real-time RT-PCR assay. The initial amount of the virus copies was determined by SDS software, version 2.0 (ABI Company). The neutralizing activity of M1A was quantified by comparing the numbers of virus copies between the virus control group and test group. As shown in Table 4, the number of virus copies for the test group dropped much more than that for the virus control group from the second day to the sixth day after virus infection. The number of virus copies decreased 200 times more in the M1A test group than in the control group on the second day after virus infection, indicating the neutralizing capacity of M1A.
TABLE 4.
Real-time RT-PCR assay-based comparison of virus copy number between virus-treated group and M1A test groupa
| Time | No. of PFU for the following groupb on the indicated day
|
|
|---|---|---|
| Control group | Test group | |
| Day 1 | 26.54 | 12.37 |
| Day 2 | 561.72 | 27.45 |
| Day 3 | 4,926.42 | 24.52 |
| Day 4 | 1,955.57 | 22.12 |
| Day 5 | 1,843.65 | 33.27 |
| Day 6 | 2,430.61 | 91.35 |
Control group and test group RNAs were collected and subjected to real-time RT-PCR on days 1 to 6 after virus infection. Initial numbers of virus copies were calculated from the standard curve.
Control group, cells treated with 100 PFU SARS-CoV; test group, cells treated with 100 PFU SARS-CoV and 0.5 mg/ml M1A.
DISCUSSION
The highly infectious disease SARS, which has a high mortality rate, is caused by a novel coronavirus, SARS-CoV. No effective anti-SARS drugs, vaccines, or therapies are available so far. Since SARS-CoV has brought great danger to human beings, the development of human anti-SARS monoclonal antibodies would provide useful passive immunity for the treatment of SARS patients (14, 24).
The phage display technique has become a powerful tool for generation of human antibodies recently. It is easy to manipulate and also has the advantage of a high selection capacity. Our studies demonstrate that it is a feasible and efficient way to obtain both rare antibodies and completely humanized antibodies directly (1, 2, 18).
In this study, strain Beijing 01 was used as the antigen for panning, since it is the most prevalent serotype in China. It has been reported that the neutralizing epitope of SARS-CoV is located mainly on the S protein; however, the amount of S proteins on viruses is very small compared with the whole virus structure protein (10, 12, 14, 15, 20, 22, 23, 24), so it is more difficult to obtain MAbs with neutralizing capacity than nonneutralizing MAbs. This problem requires some modifications of the panning and screening processes. In the present study, the lymphocyte for construction of an anti-SARS-CoV Fab library was derived from convalescent SARS patients, in whom protective titers of IgG to SARS-CoV were higher than 1:264 and 1:64 as detected by ELISA and MNT, respectively. Also, we used the highly purified virion for the first two rounds of panning and the recombinant spike protein for the last two rounds in order to increase the possibility of selecting out neutralizing Fab clones from the Fab library.
Our ELISA data confirmed that the purified M1A bound specifically to S protein. The neutralizing test demonstrated that 0.25 mg/ml of M1A could postpone the occurrence of CPE for 2 days upon challenge with 100 PFU of SARS-CoV. The titration end point of M1A was 0.5 mg/ml, which completely neutralized the activity of SARS-CoV. Although the 50% inhibitory concentration (IC50) of M1A could not be precisely determined by the MNT, it could be estimated from the MNT result that the IC50 of M1A ranged from 0.25 mg/ml to 0.5 mg/ml.
Real-time fluorescence RT-PCR was utilized to test the difference in the number of SARS-CoV copies between the group treated with 0.5 mg/ml M1A and the virus control group. This study showed that the number of virus copies for the M1A-treated group was 20 times lower than that for the control group after virus infection for 1 to 5 days. M1A displayed significant neutralizing capacity against SARS-CoV in vitro, indicating the possibility of generating a therapeutic antibody for SARS.
Previous studies have demonstrated that Fab molecules could not have a protective capacity in vivo in the absence of the Fc region. The affinity constant of whole-molecule IgG with its antigen can improve 100 times compared with that of Fab or scFv molecule forms (3, 4). Therefore, structural modifications of M1A may increase its binding affinity and neutralizing capacity. This study showed that M1A will be useful for studies on the conformational structures and immunogenicity of S protein and will furthermore be valuable for passive immunoprophylaxis against SARS-CoV.
Acknowledgments
We thank Shan Lu (Laboratory of Nucleic Acid Vaccines, University of Massachusetts Medical School) for providing the recombinant S protein for screening of the Fab library and detection of Fab binding activity.
This work was supported by national “863” grant 2003AA208204 from the National Science and Technology section.
REFERENCES
- 1.Barbas, C. F., III, A. S. Kang, R. A. Lerner, and S. J. Benkovic. 1991. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc. Natl. Acad. Sci. USA 88:7978-7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbas, C. F., III, E. Bjorling, F. Chiodi, N. Dunlop, D. Cababa, T. M. Jones, S. L. Zebedee, M. A. Persson, P. L. Nara, E. Norrby, and D. R. Burton. 1992. Recombinant human Fab fragments neutralize human type 1 immunodeficiency virus in vitro. Proc. Natl. Acad. Sci. USA 89:9339-9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Begum, N., S. Horiuchi, Y. Tanaka, N. Yamamoto, K. Ichiyama, and N. Yamamoto. 2002. New approach for generation of neutralizing antibody against human T-cell leukaemia virus type-I (HTLV-I) using phage clones. Vaccine 20:1281-1289. [DOI] [PubMed] [Google Scholar]
- 4.Bjorling, E., E. von Garrelts, A. Morner, M. Ehnlund, and M. A. Persson. 1999. Human neutralizing human immunodeficiency virus type 2-specific Fab molecules generated by phage display. J. Gen. Virol. 80:1987-1993. [DOI] [PubMed] [Google Scholar]
- 5.Burgoon, M. P., R. A. Williamson, G. P. Owens, O. Ghausi, R. B. Bastidas, D. R. Burton, and D. H. Gilden. 1999. Cloning the antibody response in humans with inflammatory CNS disease: isolation of measles virus-specific antibodies from phage display libraries of a subacute sclerosing panencephalitis brain. J. Neuroimmunol. 94:204-211. [DOI] [PubMed] [Google Scholar]
- 6.Crawford-Miksza, L. K., and D. P. Schnurr. 1994. Quantitative colorimetric microneutralization assay for characterization of adenoviruses. J. Clin. Microbiol. 32:2331-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Carvalho Nicacio, C., Å. Lundkvist, K. B. Sjölander, A. Plyusnin, E.-M. Salonen, and E. Björling. 2000. A neutralizing recombinant human antibody Fab fragment against Puumala hantavirus. J. Med. Virol. 60:446-454. [DOI] [PubMed] [Google Scholar]
- 8.Enserink, M. 2004. Infectious disease. One year after outbreak, SARS virus yields some secrets. Science 304:1097. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher, T. M., and M. J. Buchmeier. 2001. Coronavirus spike proteins in viral entry and pathogenesis. Virology 279:371-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guillen, J., A. J. Perez-Berna, M. R. Moreno, and J. Villalain. 2005. Identification of the membrane-active regions of the severe acute respiratory syndrome coronavirus spike membrane glycoprotein using a 16/18-mer peptide scan: implications for the viral fusion mechanism. J. Virol. 79:1743-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He, Y., Y. Zhou, P. Siddiqui, and S. Jiang. 2004. Identification of immunodominant sites on the spike protein of severe acute respiratory syndrome (SARS) coronavirus: implication for developing SARS diagnostics and vaccines. J. Immunol. 173:4050-4057. [DOI] [PubMed] [Google Scholar]
- 12.Holmes, K. V. 2005. Structural biology. Adaptation of SARS coronavirus to humans. Science 309:1822-1823. [DOI] [PubMed] [Google Scholar]
- 13.Ito, W., and Y. Kurosawa. 1993. Development of an artificial antibody system with multiple valency using an Fv fragment fused to a fragment of protein A. J. Biol. Chem. 268:20668-20675. [PubMed] [Google Scholar]
- 14.Li, F., W. Li, M. Farzan, and S. C. Harrison. 2005. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science 309:1864-1868. [DOI] [PubMed] [Google Scholar]
- 15.Li, Y., C. Luo, W. Li, Z. Xu, C. Zeng, S. Bi, J. Yu, J. Wu, and H. Yang. 2004. Structure-based preliminary analysis of immunity and virulence of SARS coronavirus. Viral Immunol. 17:528-534. [DOI] [PubMed] [Google Scholar]
- 16.Liang, M., M. Mahler, J. Koch, Y. Ji, D. Li, C. Schmaljohn, and F. K. Bautz. 2003. Generation of an HFRS patient-derived neutralizing recombinant antibody to Hantaan virus G1 protein and definition of the neutralizing domain. J. Med. Virol. 69:99-107. [DOI] [PubMed] [Google Scholar]
- 17.Marks, J. D., H. R. Hoogenboom, T. P. Bonnert, J. McCafferty, A. D. Griffiths, and G. Winter. 1991. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J. Mol. Biol. 222:581-597. [DOI] [PubMed] [Google Scholar]
- 18.McCafferty, J., A. D. Griffiths, G. Winter, and D. J. Chiswell. 1990. Phage antibodies: filamentous phage displaying antibody variable domains. Nature 348:552-554. [DOI] [PubMed] [Google Scholar]
- 19.Timoney, P. J., V. P. Geraghty, A. M. Harrington, and P. B. Dillon. 1984. Microneutralization test in PK(15) cells for assay of antibodies to louping ill virus. J. Clin. Microbiol. 20:128-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang, S., T. H. Chou, P. V. Sakhatskyy, S. Huang, J. M. Lawrence, H. Cao, X. Huang, and S. Lu. 2005. Identification of two neutralizing regions on the severe acute respiratory syndrome coronavirus spike glycoprotein produced from the mammalian expression system. J. Virol. 79:1906-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang, W.-K., T.-L. Sung, Y.-C. Tsai, C.-L. Kao, S. M. Chang, and C. C. King. 2002. Detection of dengue virus replication in peripheral blood mononuclear cells from dengue virus type 2-infected patients by a reverse transcription-real-time PCR assay. J. Clin. Microbiol. 40:4472-4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang, Z. Y., H. C. Werner, W. P. Kong, K. Leung, E. Traggiai, A. Lanzavecchia, and G. J. Nabel. 2005. Evasion of antibody neutralization in emerging severe acute respiratory syndrome coronaviruses. Proc. Natl. Acad. Sci. USA 102:797-801. (First published 10 January 2005; doi: 10.1073/pnas.0409065102.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yi, C. E., L. Ba, L. Zhang, D. D. Ho, and Z. Chen. 2005. Single amino acid substitutions in the severe acute respiratory syndrome coronavirus spike glycoprotein determine viral entry and immunogenicity of a major neutralizing domain. J. Virol. 79:11638-11646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu, M. 2004. SARS immunity and vaccination. Cell. Mol. Immunol. 1:193-198. [PubMed] [Google Scholar]