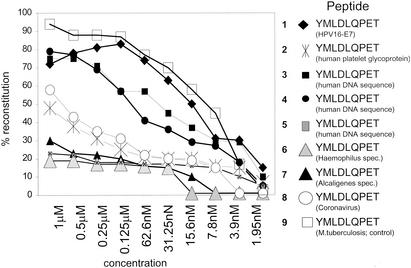
FIG. 1.
HPV16 E7 variant peptides bind to HLA-A2. Individual peptides (numbering is identical to that in Table 1) were serially diluted and tested for the capacity to reconstitute empty HLA-A2 complexes on C1R-A2 cells in the presence of human β2-microglobulin as described in detail previously (23, 50). The HPV16 E7 wild-type peptide shows high affinity to HLA-A2 compared to the coronavirus derived peptide TMLDIQPED. The high-affinity HLA-A2 binding peptide VLTDGNPPEV from an M. tuberculosis antigen (19-kDa antigen) served as the positive control. Results are expressed as percent HLA-A2 reconstitution on C1R-A2 cells. An HLA-A1 binding peptide (EADPTGHSY) derived from the melanoma-associated MAGE-1 protein did not yield detectable HLA-A2 refolding (data not shown).