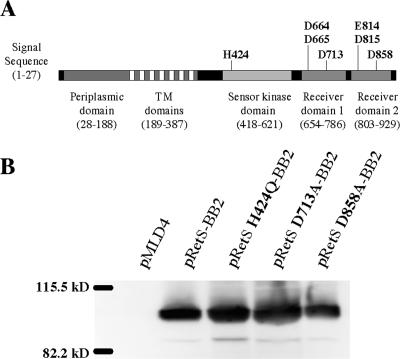
FIG. 1.
(A) Schematic representation of RetS domain structure and mutagenesis targets. retS encodes a 942-amino-acid protein predicted to contain an N-terminal signal sequence, a large periplasmic region, seven transmembrane domains, a sensor kinase domain, and two response regulator receiver domains in tandem. Residues targeted for mutagenesis are shown, with the predicted sites of phosphorylation being H424, D713, and D858. (B) Steady-state levels of RetS variants containing point mutations at the predicted sites of phosphorylation. ΔretS strains expressing untagged RetS (pMLD4), epitope-tagged wild-type RetS (pRetS-BB2), and epitope-tagged RetS containing various point mutations (pRetS H424Q-BB2, pRetS D713A-BB2, and pRetS D858A-BB2) were grown in LB-carbenicillin overnight. Bacterial pellets were lysed, and 15 μg of total protein was separated by SDS-PAGE. Proteins were transferred to a PVDF membrane and detected by Western blotting using a monoclonal anti-BB2 antibody.