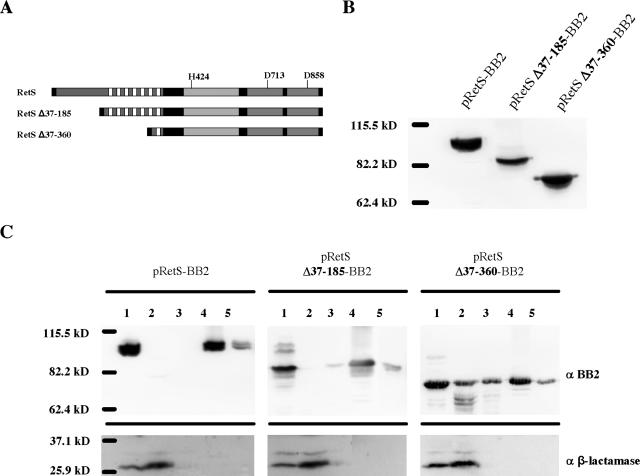
FIG. 4.
Construction of RetS truncations. (A) RetS deletion schematic. Truncations of RetS lacking either the periplasmic domain (RetS Δ37-185) or the periplasmic and six of the seven transmembrane domains (RetS Δ37-360) were constructed as described in Materials and Methods. (B) Steady-state levels of epitope-tagged RetS truncations. ΔretS strains expressing epitope-tagged alleles of wild-type RetS (pRetS-BB2), RetS Δ37-185 (pRetS Δ37-185-BB2), and RetS Δ37-360 (pRetS Δ37-360-BB2) were grown in LB-carbenicillin overnight. Bacterial pellets were lysed, and 15 μg of total protein was separated by SDS-PAGE. Proteins were transferred to a PVDF membrane and detected by Western blotting using a monoclonal anti-BB2 antibody. (C) Subcellular localization of epitope-tagged RetS truncations. ΔretS strains expressing epitope-tagged wild-type RetS (pRetS-BB2), RetS Δ37-185 (pRetS Δ37-185-BB2), and RetS Δ37-360 (pRetS Δ37-360-BB2) were grown in LB-carbenicillin to mid-logarithmic phase and fractionated as described in Materials and Methods. The lanes correspond to the indicated fractions: 1, whole-cell lysate; 2, cytoplasmic and periplasmic proteins; 3, peripheral (high-salt-extracted) membrane proteins; 4, inner membrane proteins; 5, outer membrane proteins. The three top panels were probed with anti-BB2 monoclonal antibody and show the distribution of the epitope-tagged RetS alleles; the bottom panels were probed with a polyclonal anti-β-lactamase antibody.