FIG. 1.
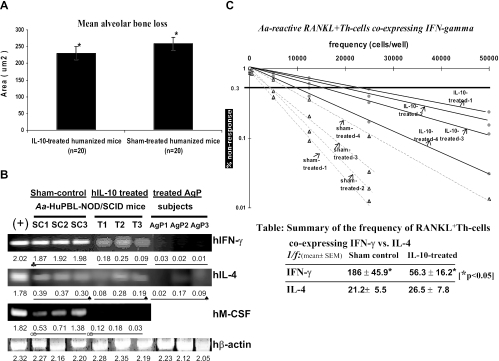
hIL-10 treatment is associated with decreased alveolar bone loss and less coexpression of hIFN-γ and M-CSF in A. actinomycetemcomitans-reactive RANKL+ Th cells in vivo. (A) There was significantly less alveolar bone loss in hIL-10-treated A. actinomycetemcomitans-infected HuPBL-NOD/SCID mice than in sham control mice at week 8 (where each autologous HuPBL sample constituted five mice each for four AgP donors, and thus, a total of 20 mice analyzed/group), as shown by statistical analysis of the difference between the two groups (means ± standard errors) (paired Student t test; P < 0.002 [*]). The data shown here are for one of two independent experiments with comparable results, for a total of 40 HuPBL-NOD/SCID mice (from four AgP donors). (B) sq-RT-PCR indicated more hIFN-γ and hM-CSF expression in A. actinomycetemcomitans-reactive RANKL+ Th cells from hIL-10-treated A. actinomycetemcomitans-infected HuPBL-NOD/SCID mice at week 8 (labeled T1 to -3) than in cells from sham control mice (labeled SC1 to -3) (n = 5 or 6 mice/HuPBL donor/group, with only three random PCR amplicons/group picked and shown here due to space limits). For hIFN-γ, P < 0.05 for SC1-3 being significantly different from the T1-3 and AgP1-3 groups; for hM-CSF, P < 0.05 for a significant difference between the SC1-3 and T1-3 groups. There was no difference in hIL-4 expression detected among the three groups (paired Student t test; P > 0.05). The positive control was concanavalin A-activated HuPBL-purified CD4+ T cells assayed at 72 h (30, 32). Extraction and purification of A. actinomycetemcomitans-reactive RANKL+ hCD4+ Th cells from periodontal tissues, sq-RT-PCR, cell preparation, and amplification of hIFN-γ and hIL-4 cDNAs were done as described previously (30, 32). For panels B and C, RANKL+ hCD4+ Th cells were purified from a total of 42 HuPBL-NOD/SCID mice, which were prepared from four AgP donors (five mice/AgP donor for three subjects and six mice for the fourth AgP donor; experiments were repeated twice). The primers used to amplify hM-CSF cDNA from study samples (labeled T1-3 and SC1-3) included the forward primer 5′-CAGATGGAGACCTCGTGCCAA-3′ and the reverse primer 5′-GCAGGCCTTGTCATGCTCTTC-3′. The PCR amplicon size for hM-CSF was 241 bp. Human β-actin was used as the internal control for PCR standardization and comparison as described previously (30, 32), with all PCR signals being quantified via LabWorks software, as reported previously (30, 32), and indicated below the gel images in panel B. The representative data shown are for one of two independent experiments with comparable results. (C) Decreased coexpression of hIFN-γ in A. actinomycetemcomitans-reactive RANKL+ Th cells after hIL-10 administration in vivo is manifested at the T-cell population level based on IF-LDA. IF-LDA was employed to assess the frequency of RANKL+ Th cells coexpressing hIFN-γ in the sham control versus IL-10-treated A. actinomycetemcomitans-infected HuPBL-NOD/SCID mice in vivo (hIL-4 results are not shown here). At weeks 7 to 8, periodontal and cervical lymph node-derived CD4+ Th cells from both groups of mice (total n = 21/group) were plated with APC and A. actinomycetemcomitans Ags for 72 h under LDA conditions as described previously (28) before being immunostained for cell surface expression of RANKL (FITC) and intracellular expression of IFN-γ (Texas Red) or IL-4 (Cy5.5) via a Leica DM-IRBE microscope with automated scanning capability and OpenLab software for quantitation of the IF signals detected (in pixels). The results showed that there was a significantly higher frequency (≅3.3×) of RANKL+ Th cells coexpressing hIFN-γ in the sham control mice than in the hIL-10-treated mice (P < 0.05; see the table in the figure for 1/f values). The resulting 1/f estimates for hIFN-γ versus hIL-4 coexpressed in FITC+ RANKL+ Th cells in both mouse groups are shown in the summary table, where asterisks depict statistical significance with P values of <0.05. The data shown are from three independent LDA experiments.