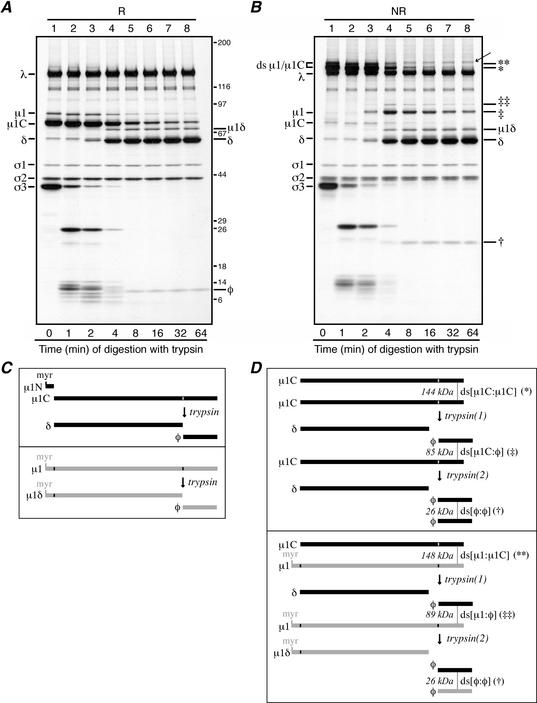
FIG. 4.
Trypsin digestions of reovirus particles. (A and B) Purified [35S]methionine-cysteine-labeled virions of reovirus T1L were treated with trypsin at 32°C. At each specified time, digestion was stopped by adding trypsin inhibitor. For the 0-min aliquot (lanes A1 and B1), trypsin inhibitor was added before trypsin. Each aliquot was then split in two, with half being mixed with reducing sample buffer (R) and half being mixed with nonreducing sample buffer (NR). Following disruption, proteins from the reduced (A) and nonreduced (B) samples were separately resolved on full-sized 5 to 20% acrylamide gradient SDS-PAGE gels and visualized by fluorography. The putative φ:φ homodimer band is indicated in panel B (†). The putative μ1C:φ and μ1:φ heterodimer bands are also indicated in panel B (‡ and ‡‡, respectively). A band of unknown origin in panel B, lanes 4 to 8, is indicated by an arrow; this band was not routinely observed in other experiments. Positions of molecular mass markers also resolved on the gel are indicated in kilodaltons in panel A. (C and D) The μ1 cleavage products are diagrammed, including the position of the intermolecular ds bond. Predicted molecular masses of the ds-bonded species are shown in kilodaltons. (C, upper box) Trypsin cleavage of the reduced μ1C monomer to yield monomeric fragments δ and φ. The myristoylated (myr) μ1N fragment arising along with μ1C from cleavage of full-length μ1 is also shown. (C, lower box) Trypsin cleavage of the reduced μ1 monomer to yield monomeric fragments μ1δ and φ. (D, upper box) Trypsin cleavage of the ds-bonded μ1C:μ1C homodimer. The necessity for nonsimultaneous cleavages of the two μ1C chains within each dimer results in an intermediate, heterodimer product that is later cleaved again. The first trypsin cleavage [trypsin(1)] yields a monomeric δ fragment and a ds-bonded μ1C:φ heterodimer. The second trypsin cleavage [trypsin(2)] then resolves the heterodimer into another monomeric δ fragmentand a ds-bonded φ:φ homodimer. (D, lower box) Trypsin cleavage of the ds-bonded μ1:μ1C heterodimer. Similar to that in panel C, the necessity for nonsimultaneous cleavages of the two chains within the dimer results in an intermediate heterodimer product that is later cleaved again. In this example, the first trypsin cleavage [trypsin(1)] acts on the μ1C chain to yield a monomeric δ fragment and a ds-bonded μ1:φ heterodimer. The second trypsin cleavage [trypsin(2)] then resolves this new heterodimer into another monomeric μ1δ fragment and a ds-bonded φ:φ homodimer. Not shown is the case in which the first trypsin cleavage acts on the μ1 chain in the μ1:μ1C heterodimer. Also not shown is the pattern of trypsin cleavage of the μ1:μ1 homodimer.