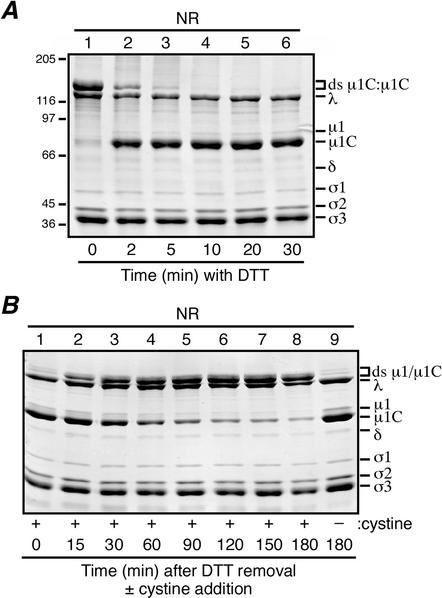
FIG. 7.
Reversibility of the virion-associated μ1/μ1C ds bonds. For each experiment, aliquots were removed from the reaction mixture at the indicated intervals, and the reaction was quenched with 50 mM IAM. When all samples had been collected for each experiment, they were mixed with nonreducing sample buffer (NR) and disrupted by boiling. Viral proteins were resolved on mini-sized SDS-8% PAGE gels and visualized by Coomassie staining. (A) DTT at a concentration of 5 mM was added to purified T1L virions and allowed to incubate at room temperature to effect in situ reduction of the ds bonds. The 0-min aliquot (lane 1) was removed before the addition of DTT. Positions of molecular mass markers also resolved on the gel are indicated to the left. (B) The ds bonds in a sample of T1L virions were reduced by DTT as in panel A, lane 6, after which the DTT was removed by dialysis. Cystine at a concentration of 5 mM was then added to promote in situ reformation of the ds bonds. The 0-min aliquot (lane 1) was removed before the addition of cystine. A sample to which cysteine was never added was also analyzed (lane 9).