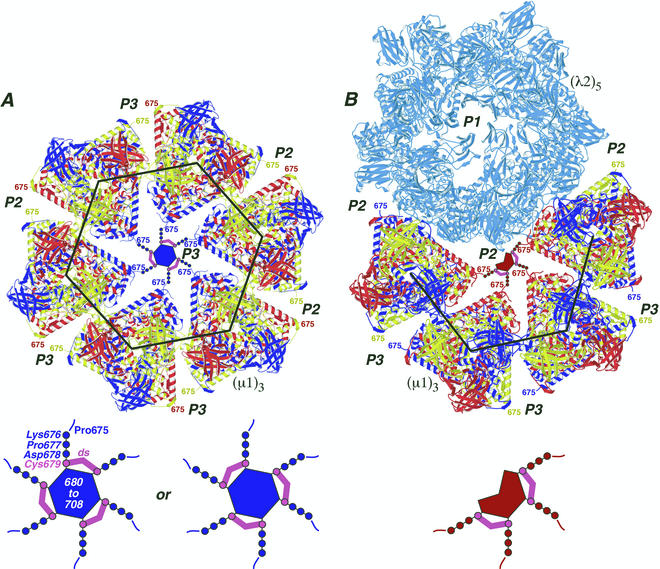
FIG. 8.
Locations of the ds bonds in the μ1 outer capsid structure. The crystal structure of μ1 was extracted from the μ1-σ3 heterohexamer complex and positioned as it appears on the surface of the virion (34). Each μ1 trimer [(μ1)3] is colored blue, red, and yellow to represent the three individual μ1 subunits. The large black hexagon and partial hexagon indicate the respectively marked regions in Fig. 1 except that the σ3 subunits are missing and the region of the partial hexagon is rotated downward by approximately 60° relative to that in Fig. 1. (A) Six μ1 trimers are shown surrounding one of the 60 P3 channels. Surrounding channels are also labeled (P2 or P3). The C-terminal residue in the μ1 crystal structure, Pro675, faces into the channels and is labeled in each of the μ1 subunits. A model for μ1 residues 676 to 708, which were not visualized in the crystal structure of the μ1-σ3 heterohexamer (34), is shown within the P3 channel that is central in this image. Cys679 and its ds bonds are shown in magenta. Residues 676 to 678 and 680 to 708 are shown in blue to match the six surrounding μ1 subunits from which they extend. Residues 680 to 708 are proposed to form the central blob evident in Fig. 1 and discussed in the text and reference 34. A magnified view of these modeled features is shown at the bottom, with labels for residues 675 to 708 and a ds bond. The alternative arrangement of the three ds bonds is shown at bottom right. (B) Four μ1 trimers and a λ2 pentamer [(λ2)5] (light blue) are shown surrounding one of the 60 P2 solvent channels. The P1 channel, which is surrounded by the five λ2 subunits and plugged by σ1 in virions (not shown), is included and labeled in this view. A model for μ1 residues 676 to 708 is shown within the P2 channel that is central in this image. Other labeling and color coding is as described for panel A, except that residues 676 to 678 and 680 to 708 are shown in red to match the four surrounding μ1 subunits from which they extend.