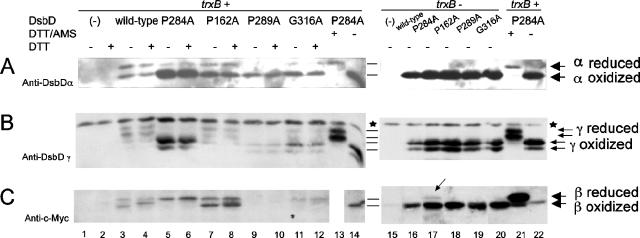
FIG. 5.
In vivo redox state of each domain in wild-type, P284A, P162A, P289A, and G316A DsbD. Proteins were detected by Western blotting, using antibodies against DsbDα (A), DsbDγ (B), and c-Myc (C). The samples in lanes 1 to 12 and 15 to 20 were precipitated with TCA and subjected to AMS alkylation. The samples in lanes 13, 14, 21, and 22 were harvested without TCA precipitation, subjected to reduction by 50 mM DTT, and precipitated with TCA. The samples in lanes 13 and 21 were subjected to AMS alkylation. The extracts were subjected to thrombin cleavage. The samples in the even-numbered lanes from 1 to 12 were subjected to reduction by 10 mM DTT before being loaded onto the gel. The strain backgrounds used were FED126 (the dsbD mutant, lanes 1 to 14, 21, 22) and FED513 (the dsbD and trxB mutant, lanes 15 to 20). The following plasmids were used: pBAD18 (−, lanes 1, 2, and 15), pSC51 (wild-type, lanes 3, 4, and 16), pSC51-10 (P284A, lanes 5, 6, 13, 14, 17, 21, and 22), pSC51-2 (P162A, lanes 7, 8, and 18), pSC51-11 (P289A, lanes 9, 10, and 19), and pSC51-14 (G316A, lanes 11, 12, and 20). Stars indicate nonspecific bands detected by anti-DsbDγ antibody, and the arrow in lane 17 indicates a small amount of the reduced β domain. The experiments were performed three times, and the results were reproducible.